| |
|
The oxidation of formaldehyde to formic acid was studied over a vanadium-titanium
oxide catalyst [Ind. Eng. Chem. Res. 28, 387 (1989).] Initially, we consider
the occurrence of only the following two reactions: |
|
| |
|
|
|
| |
|
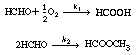
|
|
| |
|
|
|
| |
|
These reactions occur simultaneously in the gas phase. As a first
approximation, assume each reaction to be elementary. The following specific reaction
rates are with respect to HCHO 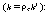 |
|
| |
|
|
|
| |
|
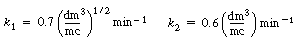
|
|
| |
|
|
|
| |
|
(a) For an entering volumetric flow rate of 100 dm3
/ min at 5 atm and 140°C, plot the molar flow rates as a function of
PFR volume. The feed is 66.7% HCHO and 33.3% O2 .
(b) Repeat part (a) for a CSTR using space time as your variable rather than
reactor volume.
|
|
| |
|
|
|
| |
|
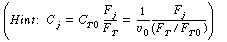
|
|
| |
|
|
|
| |
|
The space time can be varied by varying the flow rate. |
|
| |
|
(c) For a CSTR space time of 10 minutes, vary the ratio of
O 2 to HCHO in the feed. Plot
the yield of HCOOH and the selectivity of HCOOH to HCOOCH2
as a function of 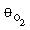
|
|
| |
|
(d) Assume that the relative values of k
1 and k 2
can be adjusted by varying temperature and catalyst composition. Vary the
rations k 1 /k
2 and simultaneously to learn their combined
effects on selectivity and HCCOH yield. Write a paragraph describing your findings.
[2nd ed. P9-13]
|
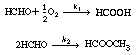
![]()
![]()