Oleic acid epoxide (E) is produced by the catalytic epoxidation of oleic acid (OA)[[J. Fotopoulos, C. Georgakis, and H. Stenger,Chem. Eng. Sci., 51, 1899 (1996)]. The raw materials are pure benzaldehyde (B) and oleic acid (OA). Unfortunately, undesired products are also formed, including benzoic (BA) and perbenzoic acids (PBA). The reaction sequence is
(1) O2 + B ![]() PBA
PBA
(2) PBA + OA ![]() E + BA
E + BA
(3) B + PBA ![]() 2BA
2BA
Additional information:
Concentration of pure B=9.8 kmol/m3
Concentration of pure
OA=3.15 kmol/m3
Catalyst concentration =4 X 10-5
kmol/m3
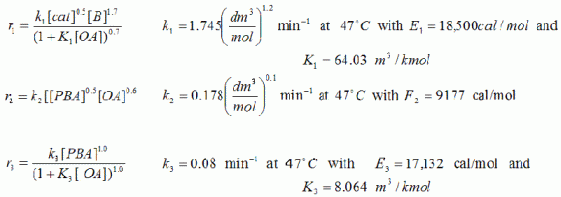
How should the reaction be carried out (e.g. type of reactor(s), volume, temperature, feed rate) to produce 100 kmol of oleic acid expoxide (E) per day? ther will be a prize for the solution that meets this criteria and minimizes the undesirable products. If equipment costs are available they should be included.