Polyesters (shirts) can be formed by the reaction of diacids and diols
nHOR1OH + nHOOCR2COOH![]() HO(R1OOCR2COO)nH +
2(n-1)H2O
HO(R1OOCR2COO)nH +
2(n-1)H2O
Let [COOH] represent the concentration of carboxyl functional groups and [-OH]
the concentration of hydroxyl groups. The feed is equilmolar in [OH] and
[COOH].
The combined mole balance and rate law is
![]()
(a) Assume that the polymerization is self catalyzed
[cat]=[COOH], plot the appropriate function of the fraction of functional groups
reacted, p, as a function of time in order to obtain a linear plot. Plot the
experimental data on the same plot. In what regions do the theory and experiment
agree, and in which region do they disagree?
(b) Assume that the
reaction is catalyzed by the (H+) ion and that the (H+ ion
is supplied by the dissociation of the weak acid [COOH]. Show that the overall
rate of reaction is 5/2 order. Plot the appropriate function of p versus time so
that the plot is linear. In which regions do the theory and experiment agree,
and which regions do they disagree?
(c) It is proposed that the
mechanism for the polymerization is
(1) 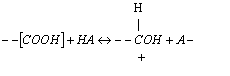
(2) 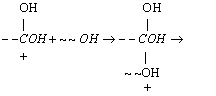
(3) 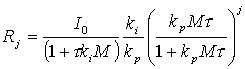
can this mechanism be made to be consistent with both rate laws?
Additional information:
Experimental Data
![]()