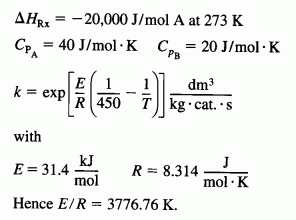A
is carried out in a packed bed reactor. There is pressure drop in the reactor and the pressure-drop coefficient is 0.007 kg-1. Pure A enters the reactor at a flow rate of 5 mol/s, at a concentration of 0.25 mol/dm3, a temperature of 450 K, and a pressure of 9.22 atm. Heat is removed by a heat exchanger jacketing the reactor. The coolant flow rate in the jacket is sufficient to maintain the ambient temperature of the heat exchanger at 27°C. The term giving the product of the heat-transfer coefficient and an area per unit volume divided by the bulk catalyst density is
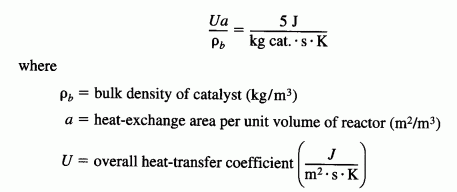
The maximum weight of the catalyst that can be packed in this reactor is 50 kg