Figure CDP12-I
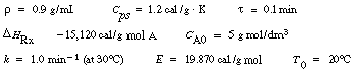
The following questions concern variation in one parameter with the others fixed at the values given.
(a) At what space-times do ignition and extinction (blowout)
occur?
(b) At what feed concentrations do ignition and extinction occur?
(c) At what feed temperatures do ignition and extinction occur? For the parameter
values given:
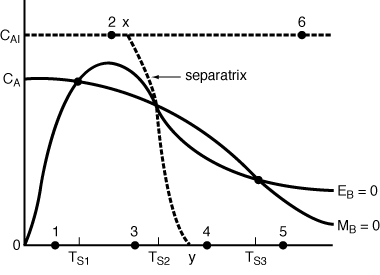
(d) Plot the curves F = 0 and G =0 in the phase space (CA , T ).
(e) Identify stable and unstable operating points, and corresponding values of
C A and T.
(f) Calculate the location of the separatrix.
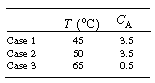
(g) Plot the phase and time trajectories for the following initial conditions:
[2nd Ed. P8-22]