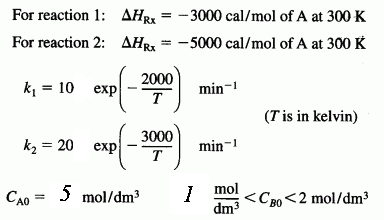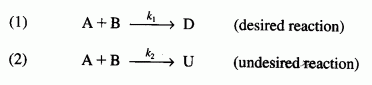
are carried out in a perfectly insulated CSTR. The desired reaction is first order in A and zero order in B, while the undesired reaction is zero order in A and first order in B. The feed rate is equimolar in A and B. Species A enters the reactor at a temperature of 100°C and species B enters at a temperature of 50°C. The operating temperature of the reactor is 400 K. The molar flow rate of A entering the reactor is 60 mol/min : CPA = 20 cal/(mol K), CPB = 30 cal/(mol K), CPD = 50 cal/(mol K), and CPU = 40 cal/(mol K).