Part C
Second Order Reaction Carried Out Adiabatically in a CSTR(c) For part (c) we will simply modify the Polymath
program we used in part (b), setting our initial temperature to 280 K. All
other equations remain unchanged.
1. CSTR Design Equation:
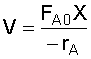
2. Rate Law:
![]()
3. Stoichiometry:
liquid,![]()
![]()
4. Combine:

Given reactor volume (V), you must solve the energy balance and the mole balance
simultaneously for conversion (X), since it is a function of temperature (T).
5. Solve the Energy Balance for X EB as a function of T:

6. Solve the Mole Balance for X MB as a function of T:
7. Plot X EB and X MB :
You want to plot
X EB and
X MB on the same graph
(as functions of T) to see where they intersect. This will tell you where
your steady-state point is. To accomplish this, we will use Polymath (but
you could use a spreadsheet).
Plot of
X EB and
X MB versus T
We see that our conversion would be about 0.75, at a temperature of 355 K.
