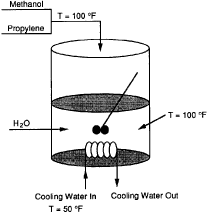
Figure CDP13-A
Propylene glycol produced by the hydrolysis of propylene oxide as discussed in Example 8-8 is to be carried out in a semibatch reactor (Figure CDP13-A). A mixture of 33.3% methanol and 66.7% propylene oxide is fed to an 80-ft3 semibatch reactor containing 30 ft3 of water at 120°F with 0.1 wt % H2SO4. The total molar feed rate is 2 lb mol/min at a

Figure CDP13-A
(a) What is the cooling water flow rate initially?
(b) What is the cooling water rate after 6 min?
You may neglect the heat of mixing. The product of the overall heat-transfer coefficient U and the heat-exchange area, A, is UA = 6 x 104 Btu/h°F.
(c) Plot the conversion and temperature as a function of time for the case of a constant cooling water flow rate of 3000 lb/h. All other conditions remain the same.