Chapter 4 Example
Production of Nitric acid
Nitric acid is made commercially from nitric oxide. Nitric oxide is produced by the gas-phase oxidation of ammonia.
4NH3 + 5O2  4NO + 6H2O
4NO + 6H2O
The feed consists of 15 mol% ammonia in air at 8.2 atm and 227°C.
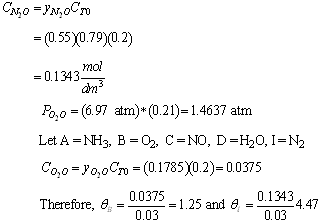
- What is the total entering concentration?
- What is the entering concentration of ammonia?
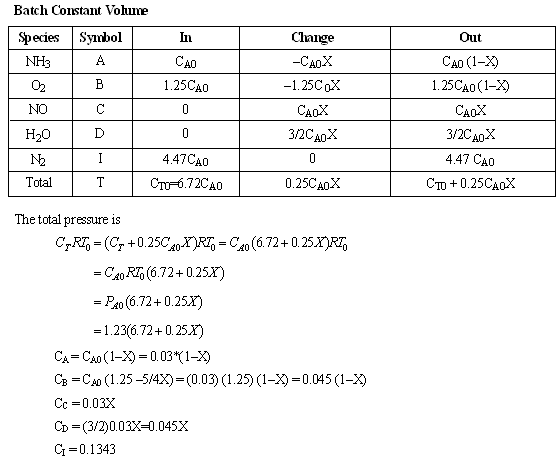
- Set up a stoichiometric table with ammonia as your basis of calculation.
- Express Ci for all species as functions of conversion for a constant-volume batch reactor. Express PT as a function of X.
- Express Pi and Ci for all species as functions of conversion for a flow reactor.
- Write the combined mole balance and rate law solely in terms of the molar flow rates and rate law parameters for C1 and C2 above. Assume the reaction is first order in both reactants
Solution
Solution
(a) Assume ideal gas behavior.
Therefore, 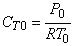
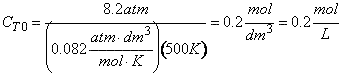
(b) Entering concentration of ammonia
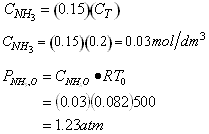
(c) Stoichiometric Table
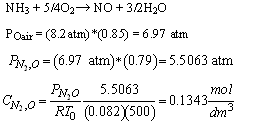
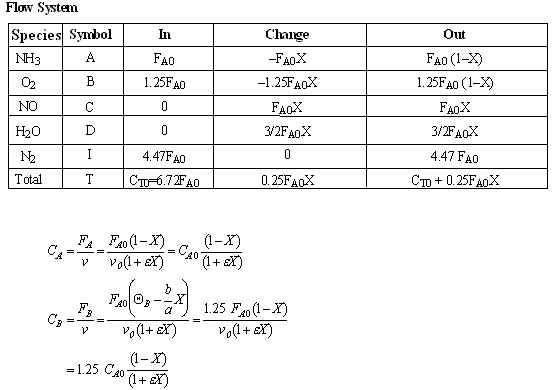
(d) Combined mole balance and rate law
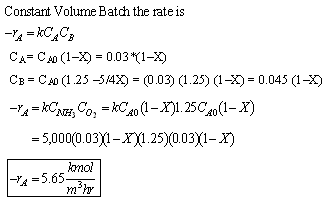
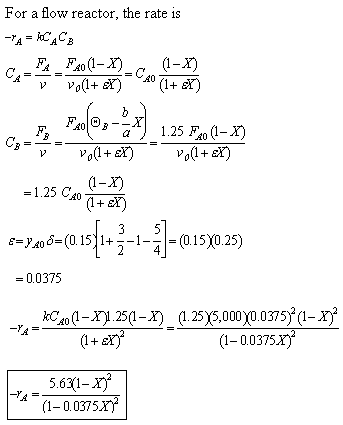
Back to Chapter 4
![]() 4NO + 6H2O
4NO + 6H2O