Write the rate law for the elementary liquid phase reaction
![]()
solely in terms of conversion. The feed to the batch reactor is equal molar A and B with CA0 = 2 mol/dm3 and kA= .01 (dm3/mol)41/s.
Hint 2: What is the concentration of A?
Hint 3: What is the concentration of B?
|
|
What is the rate law?
The reaction is elementary so what happens to the coefficients from the reaction?
Hint 2
What is the concentration of A?
Liquid phase, v = vo (no volume change)
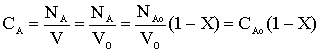
Back to Problem
Hint 3
What is the concentration of B?
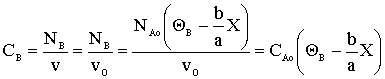
What is ΘB?
Equal molar
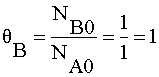
Species A is the limiting reactant because the feed is equal molar in A and B, and two moles of B consumes 3 moles of A.
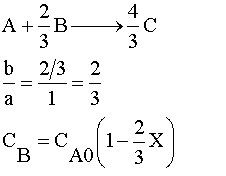
Solution
Write the rate law for the elementary liquid phase reaction
![]()
solely in terms of conversion. The feed to the batch reactor is equal molar A and B with CA0 = 2 mol/dm3 and kA= .01 (dm3/mol)41/s.
1) Rate Law: -rA=kC3AC2B
2) Stoichiometry:
Species A
Liquid phase, v = vo (no volume change)
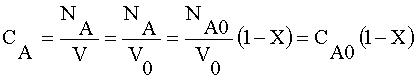
Species B
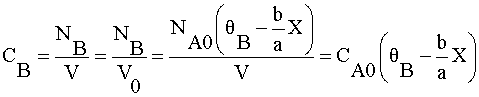
What is ΘB?
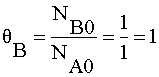
Species A is the limiting reactant because the feed is equal molar in A and B, and two moles of B consumes 3 moles of A.
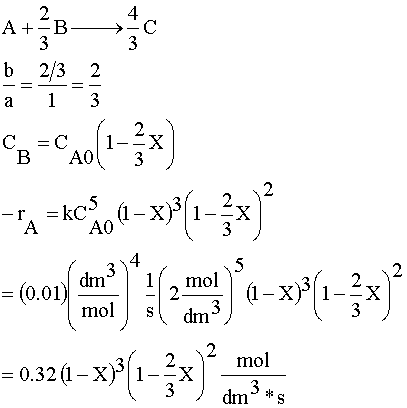
We now have -rA=f(X) and can size reactors or determine batch reaction times.