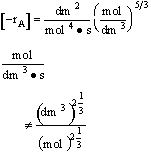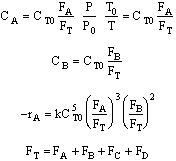Problem
The elementary gas phase reaction
![]()
is carried out in a flow reactor operated isothermally at 427°C and 28.7 atmospheres. Pressure drop can be neglected. Express the rate law and the concentration of each species as a function of conversion and as a function of the total molar flow rates. The entering volumetric flow rate is 10 dm3/s and the specific reaction rate is 200 dm12/mol4•s. The feed is equal molar in A and B.
Solution
![]()
A is the limiting reactant
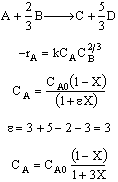
Equal molar yA0 = 1
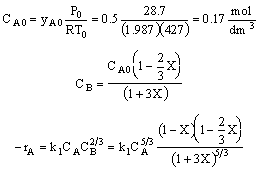
In terms of molar flow rates
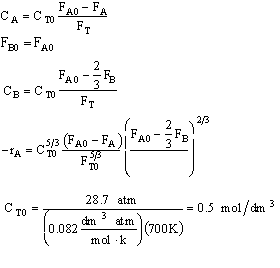
What is wrong with this solution?
1)Epsilon is calculated incorrectly. it has to be calculated on the basis of per mole of
reactant 2)CA0 is calculated incorrectly (a) the value of R is
incorrect (it was given in terms of 1.987 cal/mol•K) and (b) the temperature
substituted was in degrees Celsius rather than Kelvin 3) The rate law is incorrect the rate law is elementary as written one
cannot change it by dividing through by the stoichiometry coefficient. We could
have also known that the rate was incorrect by checking the units of k and
multiply by concentration units to the 5/3 power 4)The concentration given in terms of the molar flow rates are incorrect.
Answer
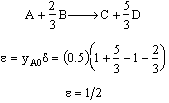
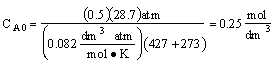
![]()