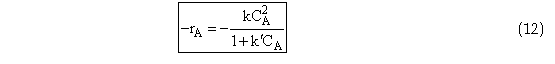Problem Statement
The rate law for the reaction
is found from experiment to be
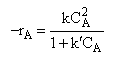
Suggest a mechanism consistent with the rate law.
Solution
Solution:
Solution
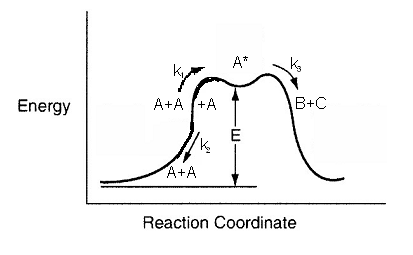
Two A molecules collide and energy is transferred from one A molecule to the other molecule making it highly reactive.
![]()
This activated molecule (A*) can do one of two things. It (A*) can collide with another molecule to become deactivated (A).
![]()
or (2) the activated molecule, A* can decompose to form B and C
![]()
For reactions with active intermediates, the reaction coordinated discussed in Chapter 3 now has trough in it and the active intermediate, A*, sit in this trough
Rate Laws
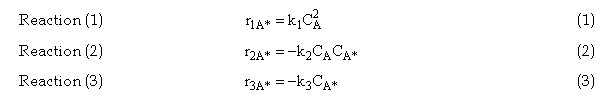
Relative Rates
![]()
Net Rates: Rate of Formation of Product
![]()
Need to find an expression for CA* because we cannot easily measure the concentration of A*, use PSSH to solve for CA*.
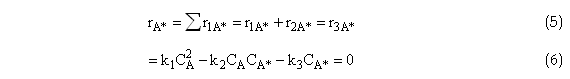
Solving for CA*
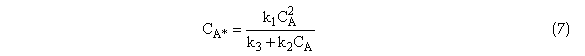
Substituting for CA* in Equation (4) the rate of formation of B is
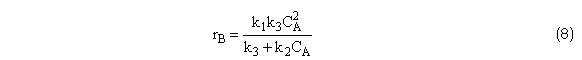
Relative rates overall
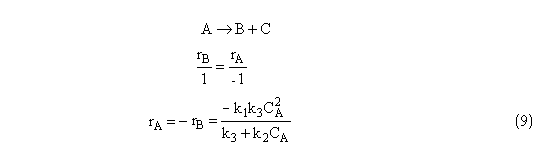
For high concentrations of A, we can neglect k3 with regard to k2CA, i.e.,
![]()
and the rate law becomes
![]()
Apparent first order.
For low concentrations of A, we can neglect k2CA with regard to k3, i.e.,
![]()
and the rate law becomes
![]()
Apparent second order
Dividing by k3 and letting ![]() and k = k1 we have the rate law we were asked to derive
and k = k1 we have the rate law we were asked to derive