Problem
The reaction
![]()
is carried out in a constant volume batch reactor. Determine the reaction order and specific reaction rate from the following data.
| t (min) | 0 | 10 | 20 | 30 |
| CA(mol/dm3) | 1 | 0.6 | 0.4 | 0.3 |
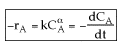
| t | CA | |||
| 0 | 1 | |||
| 0.4 | -(0.6-1.0)/(10-0)=0.04 | |||
| 10 | 0.6 | |||
| 0.2 | -(0.4-0.6)/(20-10)=0.02 | |||
| 20 | 0.4 | |||
| 0.1 | -(0.2-0.4)/(30-20)=0.01 | |||
| 30 | 0.3 |
First find![]()
| t | CA | ||
| 0 | 1 | 0.05 | |
| 0.04 | |||
| 10 | 0.6 | 0.03 | |
| 0.02 | |||
| 20 | 0.4 | 0.015 | |
| 0.01 | |||
| 30 | 0.3 | 0.005 |
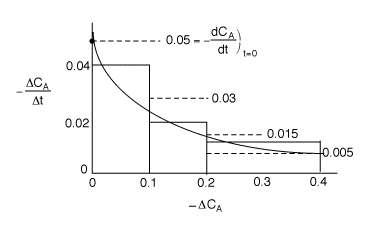
Now plot![]() versus t and it should be a straight line.
versus t and it should be a straight line.

The plot is essentially linear, therefore the reaction is zero order. From the slope of the line we
find
k=0.00167 mol/dm3 min.
What two things are wrong with this solution?
1) The graphical differentiation of the data is incorrect
as![]() is plotted as function of
is plotted as function of![]() .
First, even the data of
.
First, even the data of![]() versus
versus![]() is
not plotted correctly. According to this incorrect analysis, it should rise
linearly. However, this fact is irrelevant because the x-axis should be time,
not
is
not plotted correctly. According to this incorrect analysis, it should rise
linearly. However, this fact is irrelevant because the x-axis should be time,
not![]() . The correct plot is as follows.
. The correct plot is as follows.

| t | CA | |
| 0 | 1 | 0.053 |
| 10 | 0.6 | 0.028 |
| 20 | 0.4 | 0.017 |
| 30 | 0.3 | 0.014 |
2) The plot to determine the reaction order and reate constant is incorrect. Combined mole balance and postulated rate law is
![]()
taking the natural log of both sides
![]()
Plot![]() versus ln CA or plot
versus ln CA or plot![]() versus CA on log-log paper to determine the reaction order. The correct plot on log-log is as follows.
versus CA on log-log paper to determine the reaction order. The correct plot on log-log is as follows.

The reaction order is
![]()
therefore,

When CA=1.0 mol/dm3 then
