| Measures Other Than Conversion | top |
Uses:
A. Membrane reactors
B. Multiple reaction
Liquids: Use concentrations, I.E. CA
![]()
1. For the elementary liquid phase reaction ![]() carried out in a CSTR, where V, vo, CAo, k, and Kc are given and the feed is pure A, the combined mole balance, rate laws, and stoichiometry are:
carried out in a CSTR, where V, vo, CAo, k, and Kc are given and the feed is pure A, the combined mole balance, rate laws, and stoichiometry are:
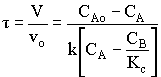
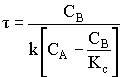
There are two equations, two unknowns, CA and CB
Gases: Use Molar Flow Rates, I.E. FI
![]()
![]()
![]()
2. If the above reaction, ![]() ,carried out in the gas phase in a PFR, where V, vo,CAo,k, and Kc are given and the feed is pure A, the combined mole balance, rate laws, and stoichiometry yield, for isothermal operation (T=To) and no pressure drop (DP=0) are:
,carried out in the gas phase in a PFR, where V, vo,CAo,k, and Kc are given and the feed is pure A, the combined mole balance, rate laws, and stoichiometry yield, for isothermal operation (T=To) and no pressure drop (DP=0) are:
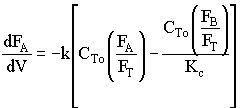
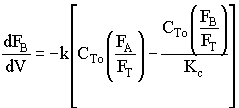
![]()
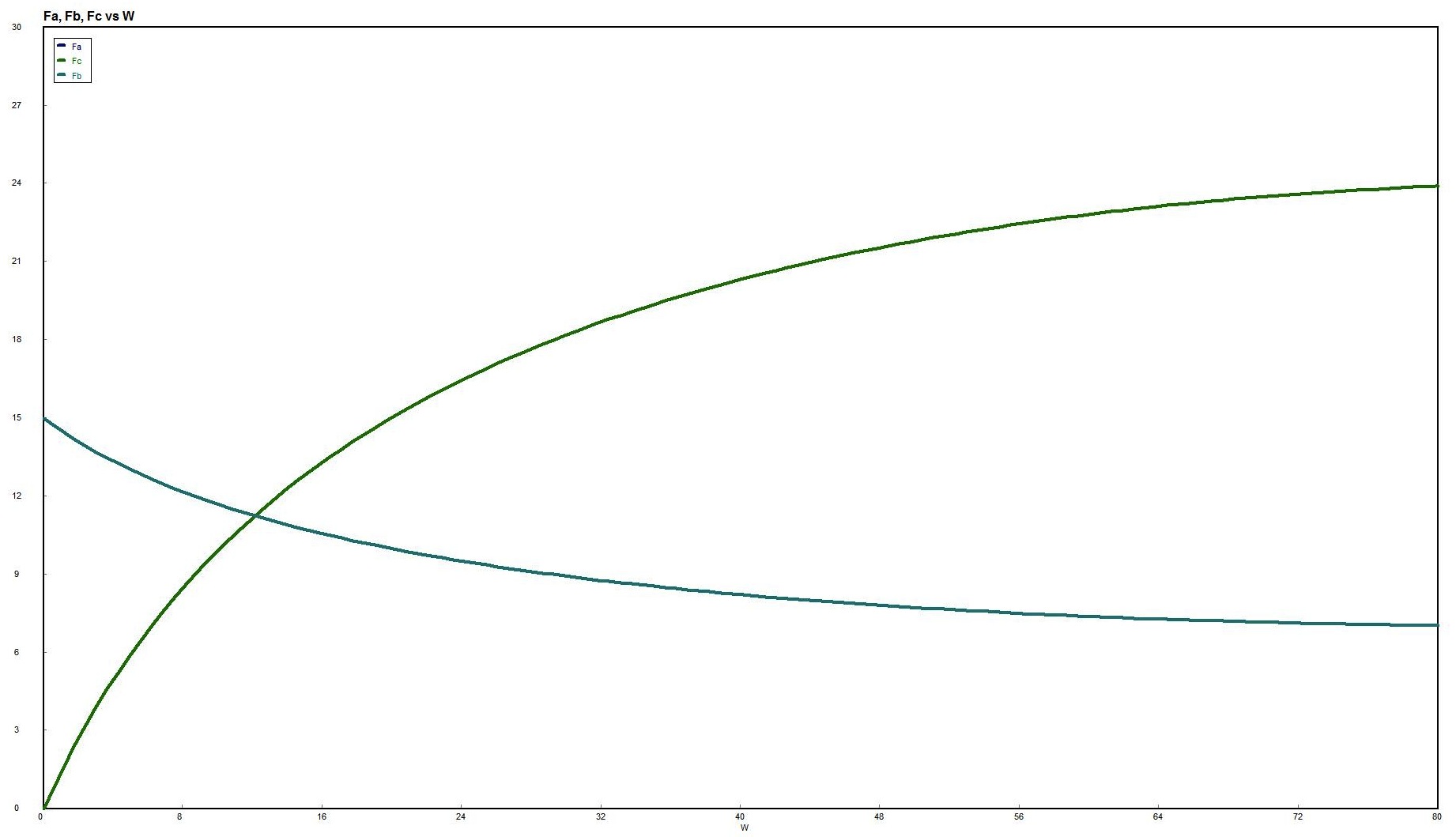
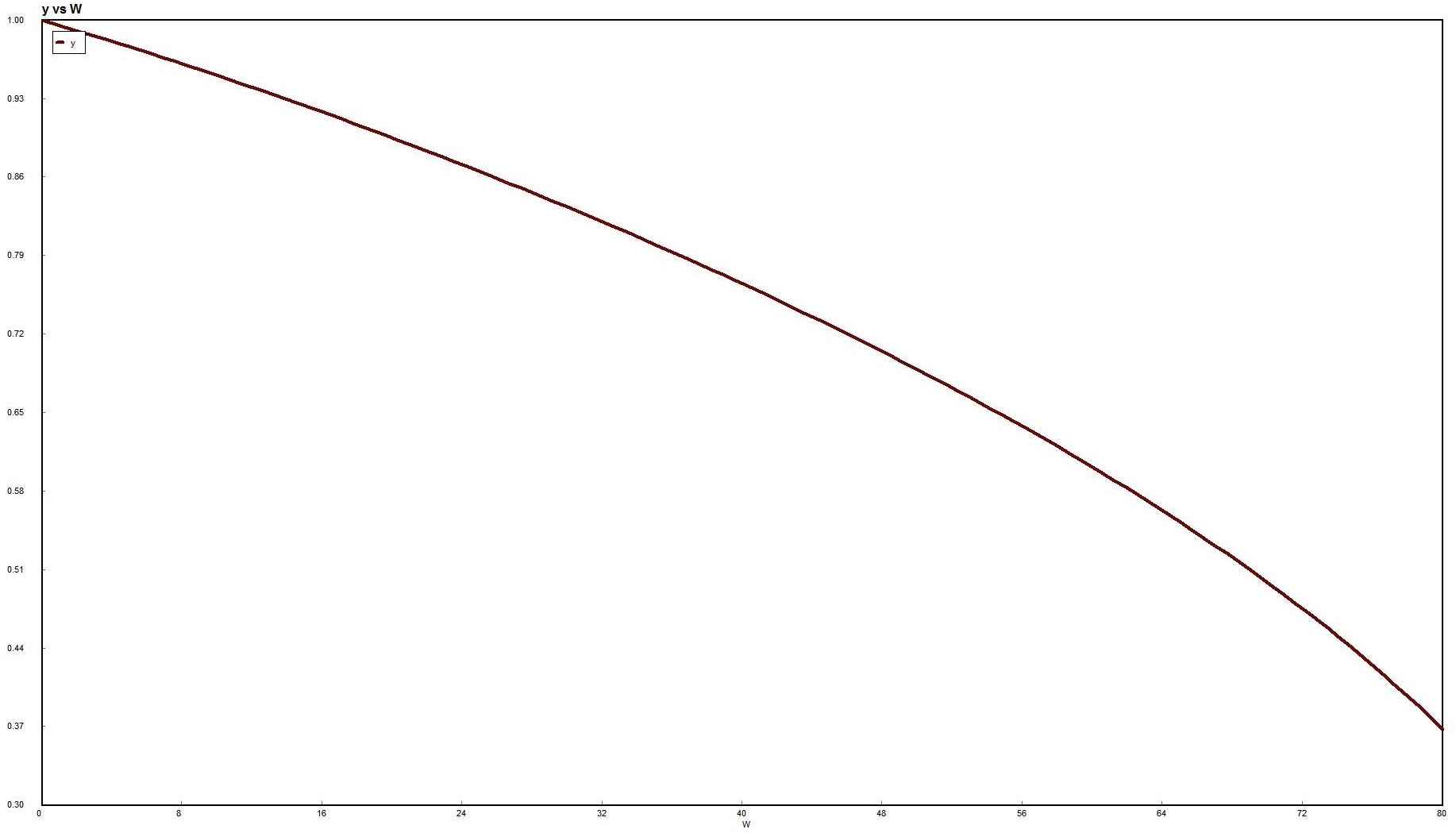
Use Polymath to plot FA and FB down the length of the reactor.
For isothermal microreactors, we use the same equations as a PFR as long as the flow is not laminar. If the flow is laminar, we must use the techniques discussed in chapter 13. See example 4.8 of the text.
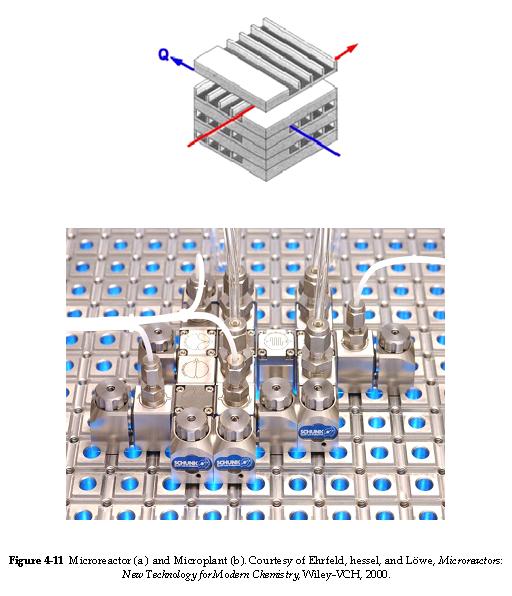
| Membrane Reactors | top |
Membrane reactors can be used to achieve conversions greater than the original equilibrium value. These higher conversions are the result of Le Chatelier's Principle; you can remove one of the reaction products and drive the reaction to the right. To accomplish this, a membrane that is permeable to that reaction product, but is impermeable to all other species, is placed around the reacting mixture.
Example: The following reaction is to be carried out isothermally in a membrane reactor with no pressure drop. The membrane is permeable to Product C, but it is impermeable to all other species.
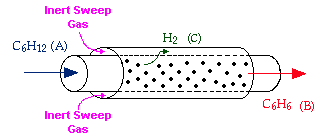 |
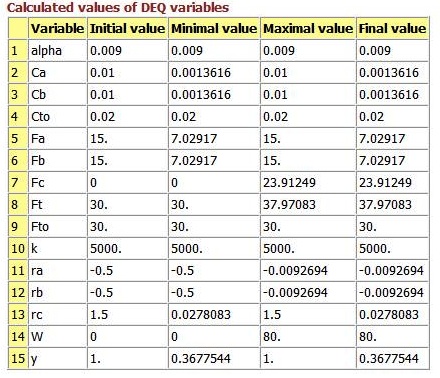
For membrane reactors, we cannot use conversion. We have to work in terms of the molar flow rates FA, FB, FC.
Polymath Program
Mole Balances |
|
Rate Laws |
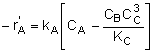 |
| |
|
| |
|
Combine |
Polymath will combine for you-- Thanks Polymath...you rock! |
Parameters |
|
Solve |
Polymath |
Here are links to example problems dealing with membrane reactors. You could also use these problems as self tests.
| Semibatch Reactors | top |
Semibatch reactors can be very effective in maximizing selectivity in liquid phase
reactions.
![]() to Selectivity
to Selectivity
The reactant that starts in the reactor is always the limiting reactant.
| 1. Molar Basis | 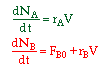 |
||
| 2. Concentration Basis | 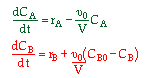 |
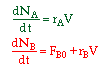 |
|
| 3. Conversion | |||
| For constant molar feed: | ||
| For constant density: | ||
Use the algorithm to solve the remainder of the problem.
Example: Elementary Irreversible Reaction
Consider the following irreversible elementary reaction:
-rA = kCACB
The combined mole balance, rate law, and stoichiometry may be written in terms of number of moles, conversion, and/or concentration:
| Conversion | Concentration | Number of Moles |
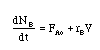 |
Polymath Equations:
| Conversion | Concentration | Moles |
d(X)/d(t) = -ra*V/Nao |
d(Ca)/d(t) = ra - (Ca*vo)/V |
d(Na)/d(t) = ra*V |
ra = -k*Ca*Cb |
d(Cb)/d(t) = rb + ((Cbo-Cb)*vo)/V |
d(Nb)/d(t) = rb*V + Fbo |
Ca = Nao*(1 - X)/V |
ra = -k*Ca*Cb |
ra = -k*Ca*Cb |
Cb = (Nbi + Fbo*t - Nao*X)/V |
rb = ra |
rb = ra |
V = Vo + vo*t |
V = Vo + vo*t |
V = Vo + vo*t |
Vo = 100 |
Vo = 100 |
Vo = 100 |
vo = 2 |
vo = 2 |
vo = 2 |
Nao = 100 |
Fbo = 5 |
Fbo = 5 |
Fbo = 5 |
Nao = 100 |
Ca = Na/V |
Nbi = 0 |
Cbo = Fbo/vo |
Cb = Nb/V |
k = 0.1 |
k = 0.01 |
k = 0.01 |
Na = Ca*V |
||
X = (Nao-Na)/Nao |
Polymath Screenshots:
| Conversion | Concentration |
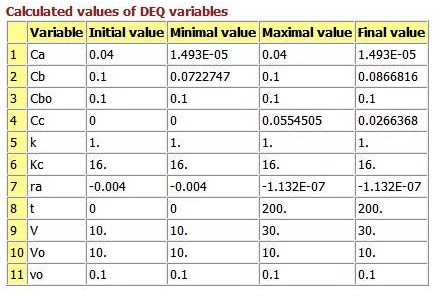
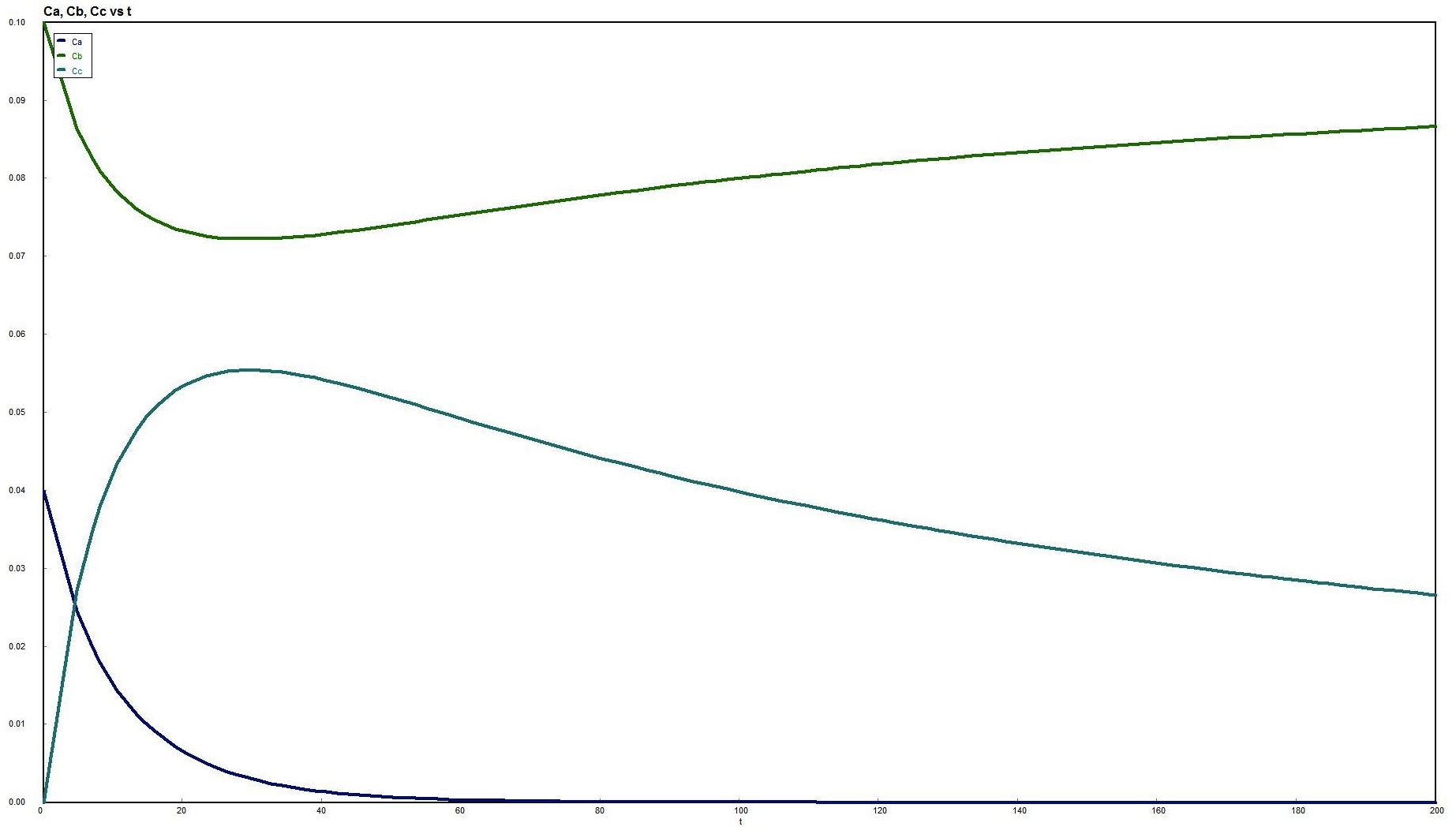
Consider the following reversible reaction:
Everything is the same as for the irreversible case, except for the rate law:
At equilibrium, -rA=0, then
![]()
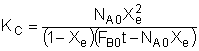
See Also:
![]() Web Module on Reactive Distillation
Web Module on Reactive Distillation
![]() You Rate Some Wetlands Critical Thinking Questions
You Rate Some Wetlands Critical Thinking Questions

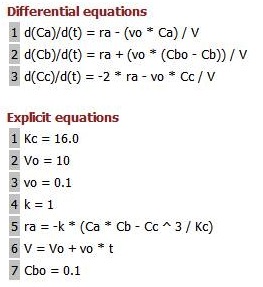
* All chapter references are for the 1st Edition of the text Essentials of Chemical Reaction Engineering .