
 |
|
By definition the concentration of A is the flow rate of A over the volumetric flow rate :
Since we are dealing with a gas the volumeteric flow rate will change down the PFR according to the equation:
The total flow rate, FT, is equal to the intial flow rate plus the change in flow rate as the reaction occurs :
Substituting in and simplifying :
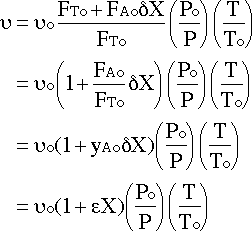
We will assume that there is no pressure drop and that it is isothermal since no information is given to the contrary:
The flow rate of A at any length along the PFR is the entering flow rate minus the amount converted:
Substituting both equations into the original gives:
![]() Back to previous page
Back to previous page