Overview
The following functionality is currently used only for the review of Data Use Agreement UFAs.
ORSP will determine if Data Office for Clinical & Translational Research (UMMS Data Office) review is required. If the study team indicates that clinical data will be shared, the Data Use Agreement will be routed to Data Office for Clinical & Translational Research (UMMS Data Office) for review.
Navigation
Role: UMMS Data Office > Home Workspace
Step-by-Step Process
- Click the UMMS Data Office role.
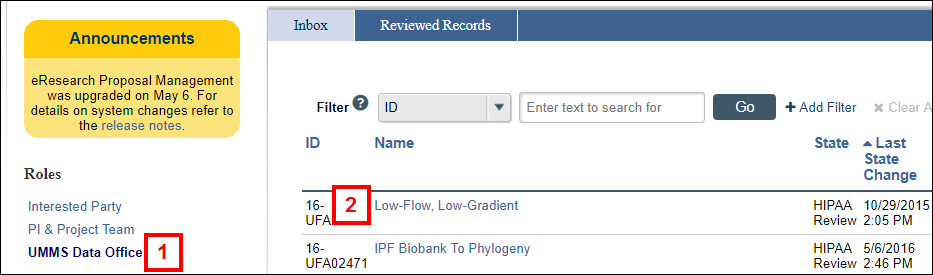
- Click the Name of the UFA.
Note The UFA is in the HIPAA Review State.
The following views are available to help facilitate your review:
- Display UFA Summary
- View UFA Worksheet
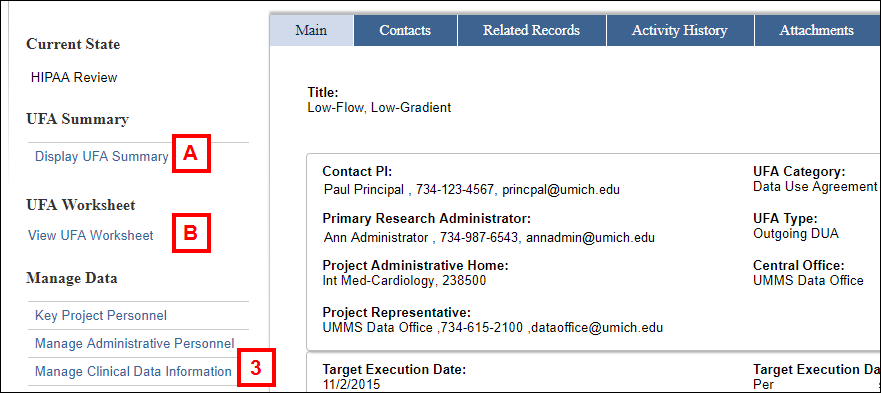
- Click Manage Clinical Data Information from the Manage Data menu.
- Complete each section of the form by clicking the applicable radio buttons and checkboxes.
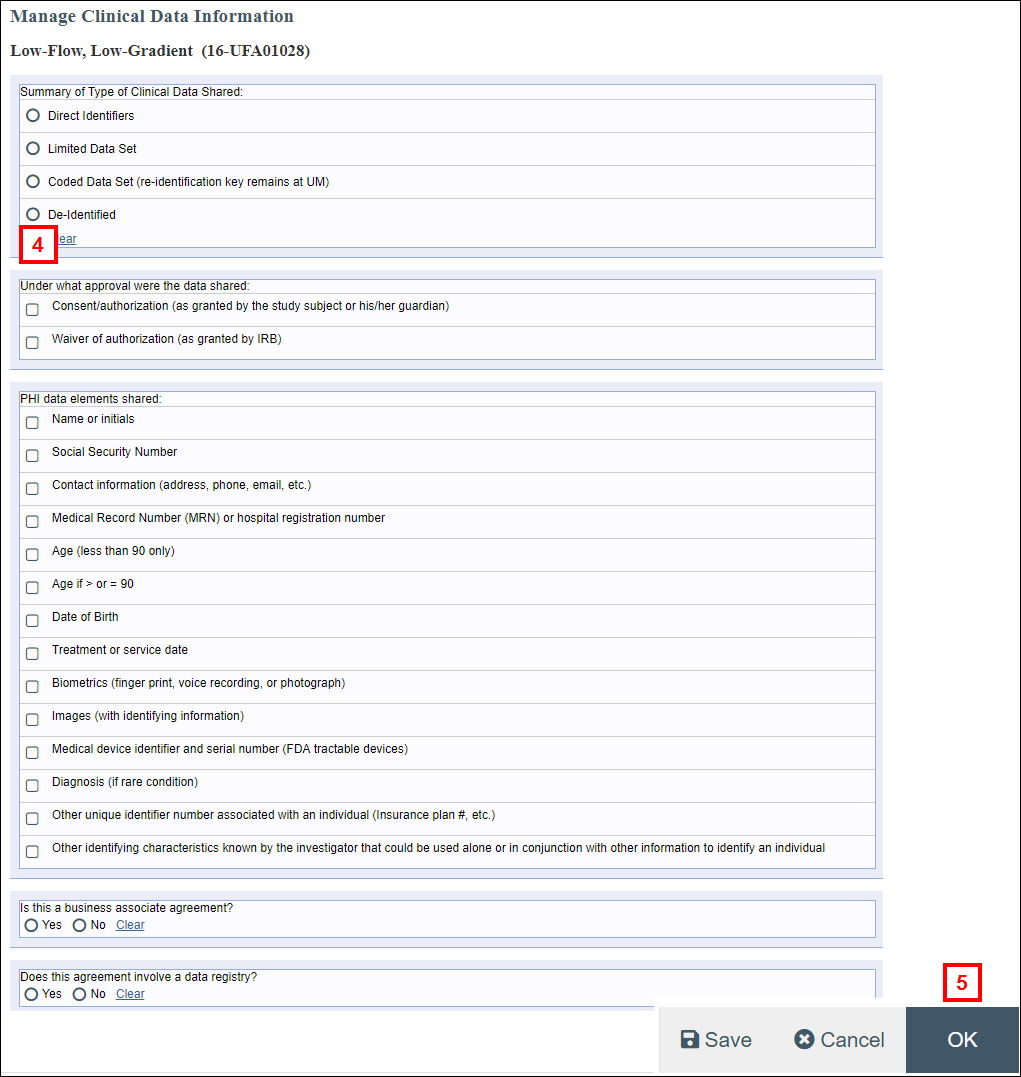
- Click OK to save your changes and return to the UFA workspace.
Note Manage Clinical Data Information can be executed multiple times as needed while completing the review. - Click the Complete HIPAA Review activity.
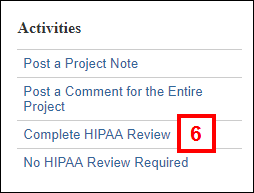
- Complete the form that displays by clicking the applicable radio buttons.
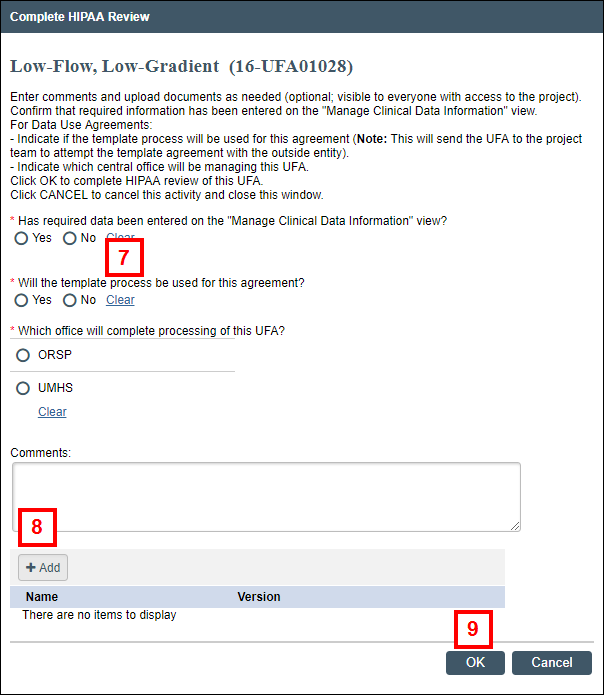
- If applicable, enter Comments or click Add to attach any documents.
- Click OK.
If a template is not being used, the UFA will route to the office you've indicated as the one that will complete processing of the UFA.
If a template is being used, the project team will receive an email notification containing a link to the appropriate template and informing them that they are responsible for obtaining a signature from the outside entity and submitting the template agreement.
