|
function places the protein component in
the proximity of the active site and suggests that the active site occurs
at the RNA/protein interface.
This is the first case where the RNA and protein components both
contribute to the molecular recognition properties of an enzyme.
Furthermore, the positions of key metal ions have been identified using
phosphorothioate substitution studies.
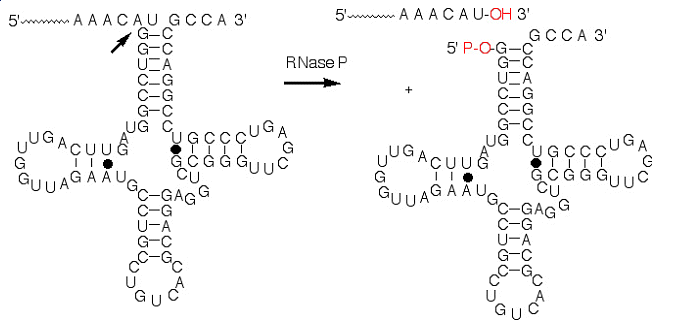
We have
further investigated the structure of the RNase P protein and complexes of
the protein with precursor tRNA segments and P RNA by utilizing both
UV-crosslinking and chemical affinity to enhance low resolution models of
the biologically active complex for comparison with the high resolution
structures. We have initiated
the kinetic analysis, using both radiological and fluorescent assays (in
conjunction with the Dr. Nils Walter's lab here at U of M), of wild-type
and variant forms of both the P protein and P RNA subunits for the
systematic dissection of the mechanism of catalysis employed by RNase
P. We have an on-going
collaboration with Dr. David Engelke's lab here at U of M to probe
RNA-protein interactions within the more complex S. cerevisiae RNase P
holoenzyme. Recently, we have
started to probe the mechanism of catalysis of the mitochondrial RNase P, a
putative protein-only complex.
PubMed
Search (most recent publications)
|