Devaux, J. F.; Hanna, I.; Lallemand, J. Y. J. Org. Chem. 1993, 58, 2349–2350.
Guevel, R. Ph.D. Thesis, The Ohio State University, 1994.
Devaux, J. F.; Hanna, I.; Lallemand, J. Y.; Prange, T. J. Chem. Res. Synth. 1996, 32–33.
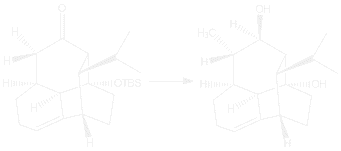
The referenced article for the third step of the mechanism (Devaux, J. F.; Hanna, I.; Lallemand, J. Y.; Prange, T. J. Chem. Res. Synth. 1996, 32–33.) involves the synthesis of a tricyclic vinigrol derivative that is similar to the vinigrol synthesized for the original article. The mechanism described in the article also utilized a similar process of adding a hydride ion to a ketone group to obtain a specific diastereomer intermediate, except the article used lithium aluminum hydride instead of Evan’s Me4NBH(OAc)3.
Three other articles that cited this reference: |
1.) Morton, J. G. M.; Kwon, L. D.; Freeman, J. D.; Njardarson, J. T. Tetrahedron Lett. 2009, 50, 1684-1686.

| These other papers contain the addition of a nucleophilic hydride ion as described in the third step of the mechanism. The first paper utilizes a similar ketone reduction reaction with sodium borohydride instead of Evan’s Me4NBH(OAc)3 to give an alcohol. |
2.) Devaux, J.; Hanna, I.; Lallemand, J. J. Org. Chem. 1997, 62, 5062-5068.

The second paper involves the reduction of a ketone with lithium aluminum hydride.
3.) Morton, J. G. M.; Kwon, L. D.; Freeman, J. D.; Njardarson, J. T. Synlett 2009, 1, 23-27.

The third paper utilizes sodium borohydride, a ketone reducing agent. |