Mechanism
H NMR Correlation 1
H NMR Correlation 2
Experimental
Leading Question
References
About Us
Homepage
|
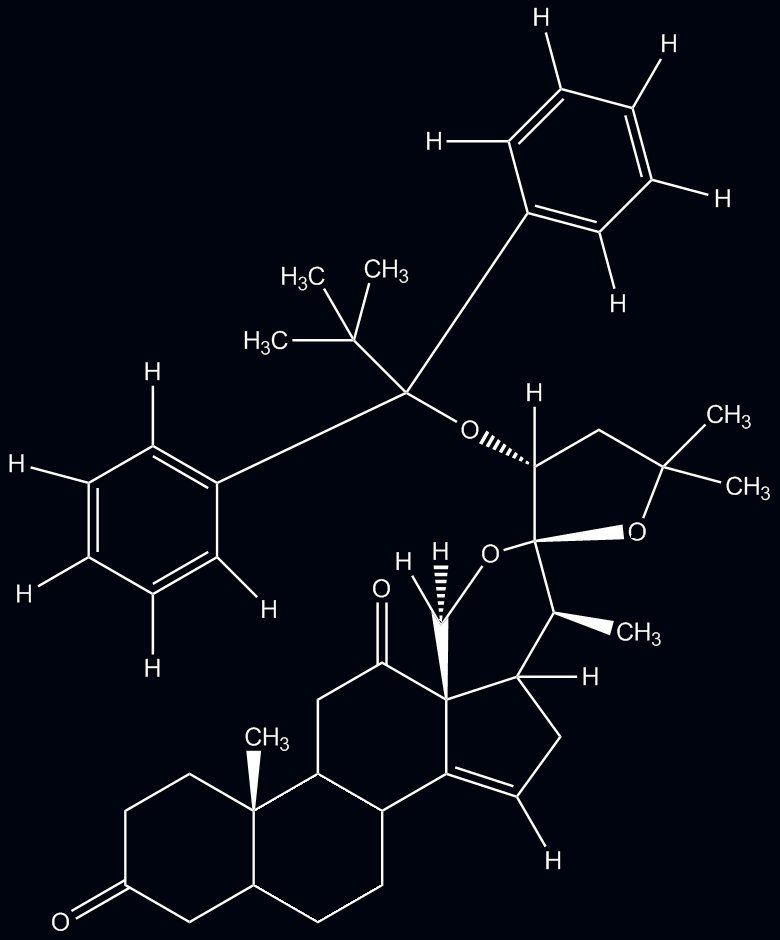 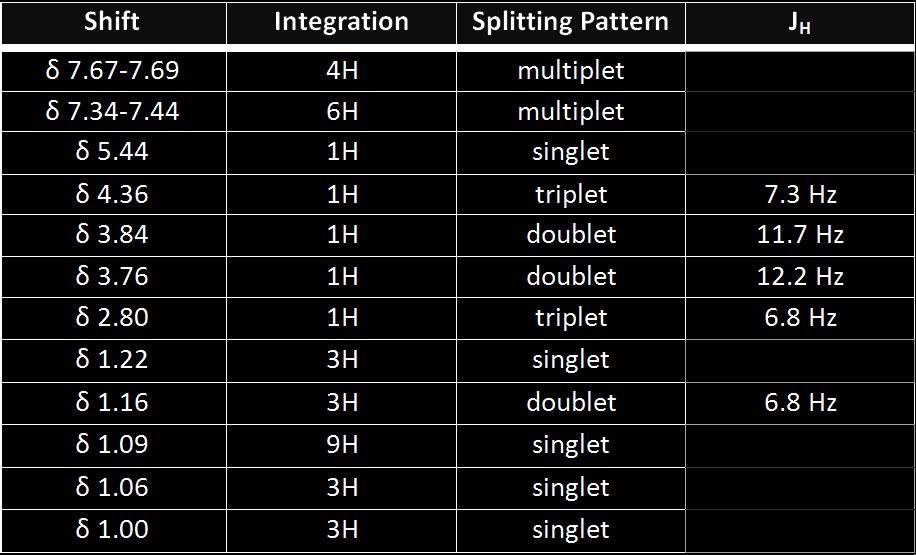
- The first peaks, 7.67-7.69 ppm, correspond to a multiplet of hydrogens located on the symmetrical benzene rings. They are more deshielded than the other set of hydrogens on the ring due to electron withdrawing effects of nearby oxygen.
- The second peaks, 7.34-7.44 ppm, correspond to another multiplet of hydrogens on the benzene rings, more shielded than their counterparts in a.
- The third peak at 5.44 ppm corresponds to a hydrogen attached to the double bond of a pentene ring. The electron density provided by the double bond shields the hydrogen.
- The fourth peak at 4.36 ppm corresponds to a hydrogen attached to the carbon on which the OTBDPS group is attached. It is deshielded by the adjacent hydrogen; due to its 2 vicinal neighbors, it appears as a triplet
- This hydrogen at 3.84 ppm, while sharing a carbon with another hydrogen (f) has a more deshielded shift value due to its proximity to the electron withdrawing effects of an adjacent oxygen and mild inductive effects of a proximal carbonyl group. It has one neighbor and thus it forms a doublet.
- This hydrogen at 3.76 ppm is affected by similar effects as hydrogen e. However, there may be some shielding imparted by the proximity of the benzene ring as they are in same stereochemical position, thus yielding a slightly lower shift value.
- The 7th peak at 2.80 ppm corresponds to the hydrogen attached to the cyclopentene ring, opposite the double bond. This double bond provides an electron density that has a deshielding effect on the hydrogen.
- The hydrogen at shift value of 1.22 ppm corresponds to hydrogens found on the methyl group attached to the carbon adjoining 2 cyclohexane rings. These hydrogens have no neighbors and therefore appear as a singlet.
- The 9th peak corresponds to hydrogens of another methyl group with shift value of 1.16 ppm. These hydrogens have 1 vicinal neighbor thus yielding a doublet splitting pattern.
- The hydrogens at 1.09 ppm are all located on methyl groups as part of the OTBDPS substituent. These hydrogens are homotopic thus yielding the same value; furthermore, they are heavily shielded by the proximal benzene rings and therefore have low shift values. They have no neighbors and therefore appear as a singlet.
- The hydrogens at 1.06 ppm are attached to another methyl group in close proximity to an oxygen atom within the attached ring. Though it would appear that the 2 methyl groups are diastereotopic, the oxygen atom provides an electron withdrawing effect thus deshielding these hydrogens slightly more than those at l. These hydrogens have no vicinal neighbors and therefore appear as a singlet.
- These hydrogens at 1.00 ppm, like the hydrogens in k though to a greater extent, are shielded by the proximal benzene (perhaps due to anisotropy), thus resulting in a low shift value. Also form a singlet due to lack of neighbors.
|

