Experimental Procedure for the Conversion of Ketone 76 to Bromide 77
To a solution of 76 in 2-heptanone (5 mL) lithium bromide (0.18 g, 2.05 mmol, 4.0 equiv) was added at 23 °C. The mixture was heated to 120 °C and reacted at the same temperature for 12 h. After cooling the solution to 23 °C, water (10 mL) was added. The aqueous layer was extracted with ethyl acetate (15 mL×2). The combined extracts were dried over anhydrous sodium sulfate, filtered, and purified by silica gel column chromatography (gradient 5% → 10% ethyl acetate–hexanes) to give 77 as colorless oil. (0.106 g, 64%): Rf = 0.48 (30% ethyl acetate–hexanes); 1H NMR (500 MHz, CDCl3) δ 5.69 (dd, J = 6.2, 1.8 Hz, 1H), 4.44 (d, J = 4.8 Hz, 1H), 4.40 (d, J = 6.9 Hz, 1H), 3.24 (ddd, J = 18.8, 4.8, 1.8 Hz, 1H), 2.90 (d, J = 18.0 Hz, 1H), 2.75 (ddd, J = 18.8, 6.2, 1.0 Hz, 1H), 2.40 (dd, J = 13.0, 6.9 Hz, 1H), 2.39 (d, J = 18.0 Hz, 1H), 1.61 (d, J = 13.0 Hz, 1H), 1.54 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 208.5, 151.1, 118.2 (q, J = 320 Hz), 110.3, 88.6, 81.0, 46.3, 46.0, 43.6, 41.0, 30.4, 22.0; MS(ES)+ calcd for C12H12BrF3NaO5S (M+Na)+ 426.94, found 427.25.
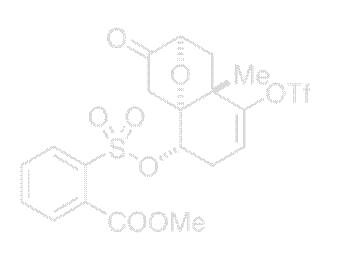
Ketone 76
|
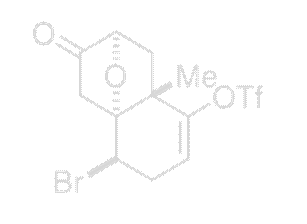
Bromide 77 |

Eric Q


