One of the Ten Commandments of Organic Chemistry is
"Thou shall not perform SN2 reactions on non-sp3-carbons."
Unfortunately, such reaction on sp2-carbons is very convinient because it would eliminate the need to protonate and then deprotonate pi bonds, a process that subjects molecules to harsh conditions and consumes large amounts of reagents and solvents. Fortunately, Stille reactions are able to maneuver around this rule.
Stille reactions involve a palladium (Pd) catalyst and an organotin compound. Pd is most stable when four other groups are attached to the atom; when used in Stille reactions, the catalyst sually has four very bulky triphenylphosphine (PPh3) ligands. The Pd-ligand catalyst usually exists as Pd(PPh3)4 but in solution it can also exist as Pd(PPh3)3 and Pd(PPh3)2.
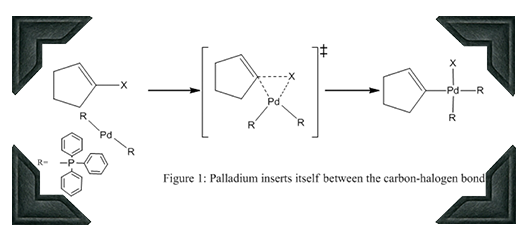
For the basic reaction mechanism, we are going to start with a halogenated cyclopentene, as shown in Figure 1. The first step of the mechanism involves the Pd catalyst, in the form of Pd(PPh3)2, inserting itself between the halogen and an sp2-carbon. This insertion mechanism is analogous to how magnesium inserts itself between brominated organic compounds to form Grinard reagents.

The resulting molecule has the halogen atom and the sp2-carbon trans to each other relative to the Pd center. This isomerization happens for several reasons. The first is that PPh3 experience large steric repulsion due to its size, so much that not they not only become trans to each other but also makes the Pd shape planar rather than a tetrahedral. The second complicated reason has to do with the electric nature of different atoms. Halogens are very electronegative, drawing electrons towards it. Carbons, however, are more willing to share their electrons. Having an electron withdrawing group and electron donating group opposite each other is more stable than having them adjacent to each other or two of the same opposite each other (note that this will come back later). The isomerization proceeds via PPh3 molecules leaving and attaching to the Pd atom, as shown in Figure 2.

The next step involves a reaction between the organopalladium molecule and an organotin molecule, say trimethylphenyltin. A concerted mechanism occurs in which the halogen and tin molecules combine and leave, resulting in Pd(PPh3)2 connected to cyclopentene and a phenyl group. At this point, the cyclopentene and benzene are trans, as shown in Figure 3.

Going back to the electric nature of atoms, there are now sp2-carbons opposite each other, meaning that the trans form of this compound is not the most stable form. Here, another isomerization occurs, as shown in Figure 4, and the sp2-carbons are cis to each other, as are the PPh3 ligands. Steric repulsion between PPh3 forces the ligands apart and makes the sp2-carbons closer to each other. They are then forced of Pd and form a new bond between the two, as shown in Figure 5.

Note that the usage of tin in the Stille reaction imposes limitations on the use of any compounds synthesized using this mechanism. Tin acts as an toxin, as it interferes with many enzymatic pathways, and is a neurotoxic compound. When tin is found in organic compounds, it is much more easily absorbed by the body than inorganic tin, so if steroids such as Cortistatin A, with potential drug usage, are synthesized using the Stille reaction, the intermediate products would require careful regulation to ensure acceptably low levels of tin in the final product.