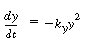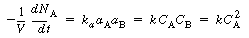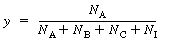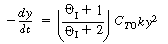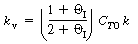| |
|
Your textbooks after your graduation will be, in part, the professional
journals that you read. As you read the journals, it is important that you study
them with a critical eye. You need to learn if the author's conclusion is supported
by the data, if the article is new or novel, if it advances our understanding, and
to learn if the analysis is current. To develop this technique, one of the major
assignments used in the graduate course in chemical reaction engineering at the University
of Michigan for the past 20 years has been an in-depth analysis and critique of a
journal article related to the course material. Significant effort is made to ensure
that a cursory or superficial review is not carried out. The students are asked to
analyze and critique ideas rather than ask questions such as: Was the pressure measured
accurately? They have been told that they are not required to find an error or inconsistency
in the article to receive a good grade, but if they do find such things, it just
makes the assignment that much more enjoyable. Beginning with Chapter 4, a number
of the problems at the end of each chapter in this book are based on the students'
analyses and critiques of journal articles and are designated with a C (e.g., P4C-1).
These problems involve the analysis of journal articles that may have minor or major
inconsistencies.
Analysis is the process of breaking a problem down into recognizable
elements (i.e., pieces) such that meaningful relationships exist between the elements.
Consequently, the first step in analyzing a problem is to identify all the elements
of the problem and clarifying them.
Finally, after classifying the elements into different groups, we need to formulate
relationships between the elements within a group and between groups. The heart of
the analysis process is the careful comparison of similarities and differences of
the elements. What distinction accounts for the differences? What is the reason for
the distinction? Look for increases or decreases between sequential elements in a
series. Seek correspondences between diverse ideas.
In analyzing and critiquing journal articles, students are asked
to identify elements of the article and classify them according to key ideas, key
assumptions, key theories, key experiments, and key results. Students then continue
to analyze the article by asking such questions as:
- What do you believe was the most difficult hurdle for the authors
to overcome?
- What is the novelty or innovation in this work?
- How will the results or techniques described in this work be useful
to other investigators?
- Can the author's results be explained by another theory or hypothesis?
- Why are the last data points always off the curve predicted by
the theoretical model?
All challenges by students of ideas and conclusions of the articles must be supported
by calculation or pertinent literature. When reading the literature, students look
for experimental points that fall off the theoretical curve and for statements or
equations that are not in a familiar form and do not look quite right. For example,
the article analyzed for Problem P4C-1 discusses the effect of pressure on a liquid-phase
reaction of the type
A + B 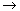 C C
The rate equation for the batch reaction was written in terms of
the mole fraction of A, y, for an equal molar feed containing an inert I:
|
|
| |
|
Consequently, it may be possible to explain all or part of the increase
in the specific reaction rate by the fact that the initial concentration, C T0 ,
increases as pressure increases, especially for a pressure increase of 6000 atm.
By learning the liquid compressibility of this or an analogous liquid, one may carry
out calculations to see if this challenge is substantiated. |
|
| |
|
Before the students write the final copies of their critiques, they
are asked to outline the challenges they intend to pursue. Many students state that
they will check the mechanism proposed by the author, check the assumptions made
(such as neglecting pressure drops), rederive the basic material balance equation,
and so on. Unless there is evidence that something looks a little "fishy,"
these types of statements are not sufficiently penetrating, as a deeper level of
questioning is required in a critique. If an assumption is to be challenged, it should
be one that would affect the maximum sensitivity of the experiment. For example,
if a 10-fold increase in the pressure would only result in a 2% increase in the rate
of reaction, it doesn't make much sense to redo the pressure-drop calculations for
a packed-bed reactor. On the other hand, if a temperature rise of 2 or 3 degrees
causes the reaction rate to increase significantly, it would be logical to check
the isothermality of the reaction bed. Finally, one should refrain from proposing
questions relating to the authors' experimental planning and techniques, since one
would not be able to resolve that without going to the laboratory and actually taking
the data. However, suggesting key experiments that would resolve conflicting theories
or reaction mechanisms is encouraged. |
|
 C
C