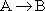
The isomerization reaction
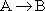
is carried out in a CSTR with a heat exchanger. We want to construct a stability diagram. First we will consider a zero order reaction with
![]()
Next, we will consider a first order reaction to be with
![]()
Construct the stability curves for a zero order reaction, (S0), and a first order reaction, (S1), as a function of TC.
Additional Information
The ratio of inerts to A fed is 2 to 1.
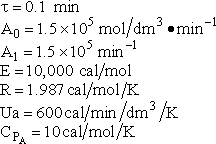 |
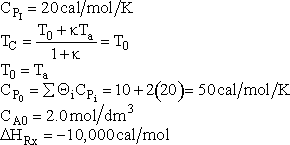 |
Solution for Zero Order Reaction
| (1) |
For X*<1: |
(2) |
Otherwise X* = 1 |
Choose TC and solve for T* using the Polymath program in Table 1.
|
(3) |
We shall use the same Polymath program shown in Table 1 to solve for S0 and S1. Note, in the Polymath program for this zero order reaction we let T1 = T* and then solve Equation (4) for T1.
|
(4) |
After solving for T1 (i.e., T*), we substitute for T1 to find X* in Equation (2) and then also into Equation (1) to find S0 at the chosen TC
|
(5) |
We can calculate the conversion (X1) in the Polymath program for this zero order reaction
|
(6) |
Provided X1 ≤ 1. Otherwise X1 = 1. Choose another TC and repeat solution (Equations (3) through (6)) to construct S0 versus TC in Table 2 which we will use to find regions of stability
Solution for First Order Reaction
Use Polymath to solve in Equation (7) for T*
|
(7) |
Note: In the Polymath program we let T* ≡ T for a first order reaction
Evaluate X* at this TC
|
(8) |
Calculate S1 at this TC
|
(9) |
The Polymath solution for TC = 475K is given below. We now choose another value of TC and repeat to find T* and then S1 to construct a table of S1 versus TC as shown in Table 2.
Table 1
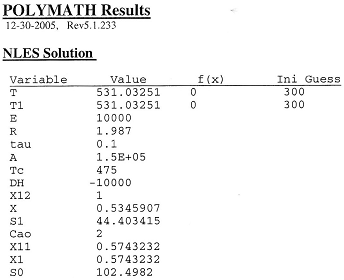

Table 2
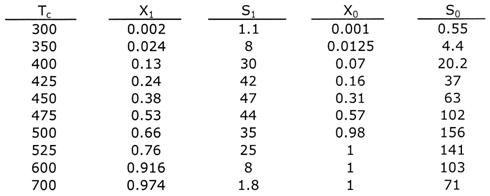
When this program is repeated for different values of TC, Figure 1 can be developed using Table 2 where both S0 and S1 are plotted as a function of TC on Figure 1.
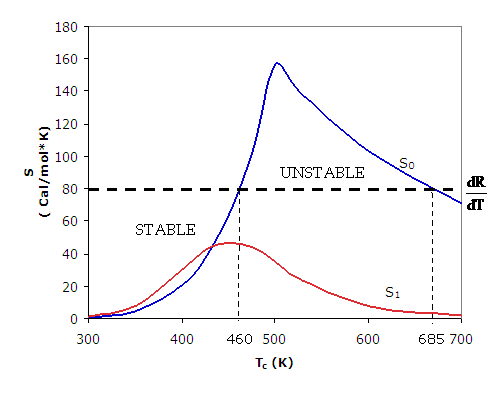
Figure 1. Stability plot.
We now can find the region of stability and of runaway
|
(10) |
Recall that
|
(11) |
then taking the derivative
|
(12) |
|
(13) |
also recall
|
(14) |
When
|
(15) |
runaway will NOT occur. However,
when ![]() (i.e.,
(i.e., ![]() )
)
runaway WILL occur.
We see that for ![]() the first order reaction is globally stable for all TC. However, we see the zero order reaction will run away when the entering temperature TC is increased above 460K. We also note the runaway reaction becomes stable again as TC is further increased to values above 685K.
the first order reaction is globally stable for all TC. However, we see the zero order reaction will run away when the entering temperature TC is increased above 460K. We also note the runaway reaction becomes stable again as TC is further increased to values above 685K.