Release Notes Version 2.8
Release Date: June 18, 2012
The following changes apply to Study Teams:
Application changes:
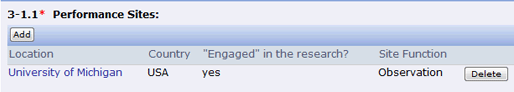
- Question 3-1.1(Performance Sites): A Site Function column has been added to better indicate the type/purpose of the site for submission review. This column also displays in the Continuing Report enrollment tables (section 2-2).
- Section 16 (Devices) has been updated to improve clarity:
- Question 16.2.12 has been reworded to highlight that the device will be used for an "off-label" or "non-approved" purpose in the study.
- Question 16.2.28 has been reworded to highlight that device will be used for "on-label" purposes in that study.
- Question 16.2.34 now asks if the device is the object of the study. If the answer is "yes", question 16.2.35 appears, allowing you to upload the product label or other supporting documentation. This change makes it easier to determine when documentation is required for a device.
IBC Registration changes:
- Question 5.2.2 (Use of Animals) now includes more specific information to help you provide the appropriate level of detail.
- You can now use the Post Correspondence activity to upload revisions to documentation for an IBC Registration. However, you cannot delete documents.
ORIO changes:
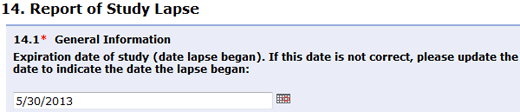
Question 14.1 (Report of Study Lapse - General Information) was updated to reflect a fix and allow you to provide more accurate information. The expiration date defaults to the current expiration date of the parent study. This field now can be edited to indicate the date the lapse began.
Other changes:
- Originally Approved HUM Application (HTML view) - Going forward, this "HTML snapshot" will look like the Printer Friendly Version of the submission. This change only impacts submissions approved after the 2.8 release. The HTML view is different from the printable version:
- View links for detail pages do not work in the HTML snapshot. Instead, the detail information is automatically displayed below the applicable section.
- Until a framework upgrade is implemented, you also may see apostrophes replaced with odd characters (e.g., ?, =).
- Accept role activity - The "accept responsibilities" checkbox has been moved to the bottom of the page for the Application or Amendment.
- Approve with Contingencies Letter - The format of the letter has been updated to improve readability.
- Fixes applied:
- The status meter now displays the correct state (Submission in Expired - Continuation in Progress) when a continuing report is filed on an expired application.
- The bug that occasionally prevented edits when an Adverse Event was returned for changes has been fixed. You can now edit the Serious Adverse Event Detail page for section 2.2.
The following changes apply to Core Staff & Committees:
Agenda & Reviewer Checklist changes:
- Updated: The agenda layout to improve legibility and display the entire title of the submission. This applies to the View Agenda by Reviewer, Submission Type, PRC, and IBC activities.
- Added: Three (3) Agenda Item type values for IRBMED: Direct or Indirect Benefit, Waiver of Documentation of Consent, and Waive/Alteration of Consent.
- Added: Biostatistician field to the PRC Agenda to show the Biostatistician named in the application.
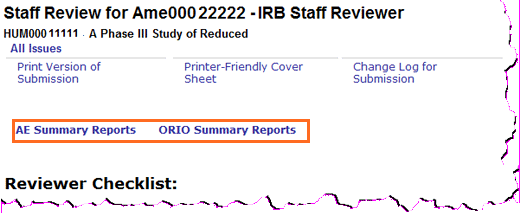
- Added: AE Summary Report and ORIO Summary Report links to the Reviewer Checklists.
Fixes applied:
- When a continuing report was approved before an ORIO, the expiration date would change. This has been fixed to display the expiration date of the study at the time the ORIO is initiated. Study teams can edit this date in the ORIO to indicate when the lapse in the study began.
- The expiration date for a Termination report will no longer be set automatically to 12 months when the reviewer completes the checklist using the Validate Expedited Decision activity.
- PRC workflow: Amendments to approved applications will now route to PRC for review when changes are made to section 8 (subject participation).
The following changes apply to IBC:
Two (2) new activities expand IBC functionality on Registrations:
- Manage Documents allows you to add, edit, and delete documents associated with an IBC Registration.
- Update Inspection Records allows you to add/maintain inspection data related to an IBC Registration.
IBC Registration editors will now be cc'd on the study team notification when the IBC Registration is approved. This change applies to the Validate Committee Decision and IBC Staff Contingency Review.