Ring Closing Metathesis?
Ring-closing metathesis (RCM) facilitates ring closure for 5, even 30, member rings that would otherwise be difficult to do due to large size. This synthesis is essentially a version of the olefin metathesis, except the reagent used is often Grubbs catalyst. Grubbs catalyst is an extremely versatile reagent that is soluble in many solvents and can support a variety of functional groups in the alkene part. Pictured below is Grubbs First Generation Catalyst:
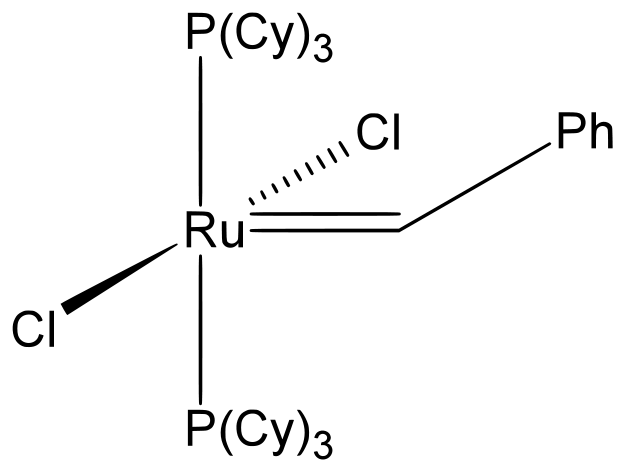
benzylidene-bis(tricyclohexylphosphine)dichlororuthenium
The general outline of the metathesis is that two molecules, both containing double bonds, switch attached groups. Cis and trans issues arise and the stereochemistry is determined by the position the molecules originally interact. An important outcome of ring-closing metathesis is the production of a cycloalkene and another alkene (generally ethene). The procedure is outlined below.
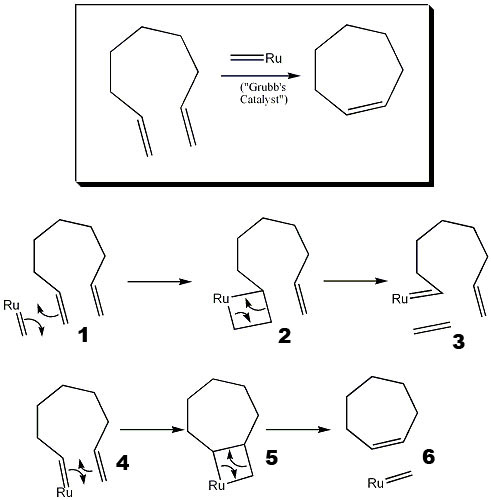
- Grubbs catalyst lies parallel to the double bond of the open ring. Both electron densities are in close proximity.
- Electrons rearrange, temporarily forming another ring, so that Grubbs reagent is part of the bigger ring.
- An alkene is let loose as a product.
- The open ring with the attached catalyst begins to lie parallel to the other double bond, the electron densities interact.
- A temporary ring forms from the electrons of the two double bonds- more rearrangement takes place as the two molecules switch groups one more time.
- Finally, with the groups switched, Grubbs catalyst is let loose, leaving a closed cycloalkene.
