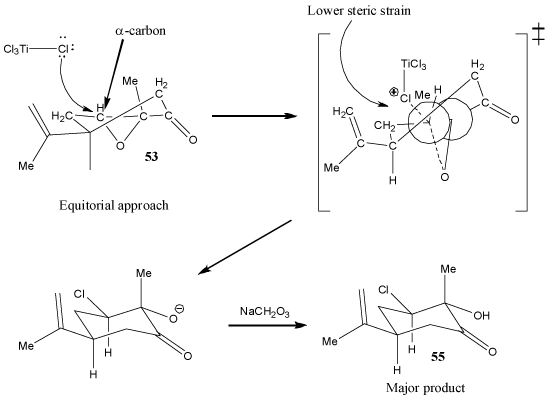
Figure 1
Citations
Related Sources:
Huet, J.; Maroni-Barnaud, Y.; Anh, N. T.; Seyden-Penne, J. Tetrahedron Lett. 1967, 17, 159-162.
Stork, G.; Stryker, J. M. Tetrahedron Lett. 1983, 24, 4887-4890.
Main Source:
Chérest, M.; Felkin, H. Tetrahedron Lett. 1968, 9, 2205-2208.

Figure 1
This source discusses the stereoselective reaction of nucleophiles with the carbonyl group of a cyclohexanone. It suggests that depending on how the nucleophile attacks the carbonyl carbon, a transitional state with either steric or torsional strain can be produced. The nucleophile will prefer to attack the face of the carbonyl carbon that produces a transitional state with a lower overall strain. In this case, though the nucleophile does not attack at the carbonyl group (it attacks at an expoxide carbon, the same principle seems to apply. Here, the nucleophilic chlorine atom from TiCl4 (a Lewis acid) prefers equatorial attack at the α-carbon (Figure 1), due to the fact that the epoxide group causes the ring to be very conformationally constrained. Therefore, axial attack of the chlorine atom would cause severe torsional strain in the transition state (Figure 2). The strain caused by steric interaction between TiCl4 and alkyl group in the transition state following equatorial approach is small by comparison. The product with an chlorine in an equatorial configuration is thus heavily favored.
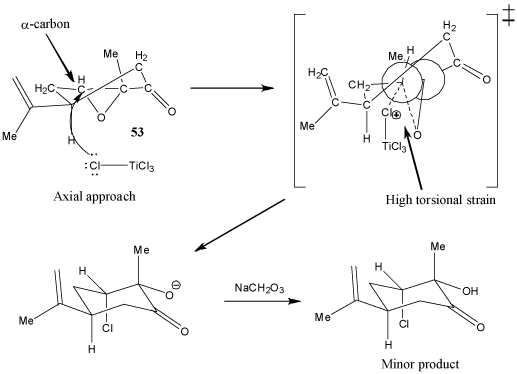
Figure 2
Other articles citing main source:
1) Breight, B.; Schmidt, Y. Chem. Rev. 2008, 108, 2928-2951.
This article uses the main source to explain why organocopper reagents attack carbonyl carbons in such a stereoselective manner.
2) Balcells, D.; Maseras, F. New J. Chem. 2007, 31, 333-345.
This article uses the main source to support computational evidence as to why nucleophiles prefer to attack a certain face of a carbonyl carbon, which determines the stereochemistry of the major product.
3) Dhotare B.; Chattopadhyay A. Tetrahedron Lett. 2005, 46, 3103-3105.
This article uses the main source to explain stereoselectivity in the addition of a nucleophile to a ketone carbon.