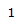
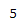
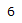
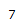
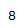
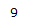
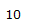
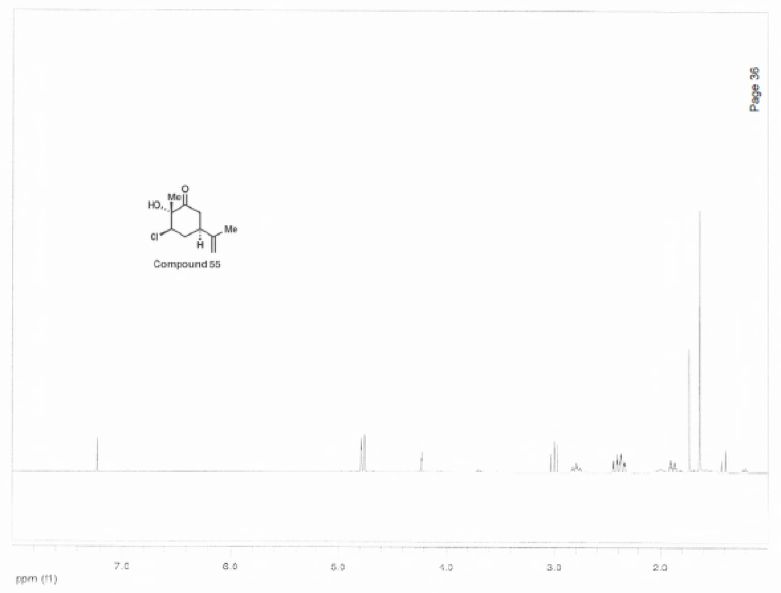
HNMR Spectroscopy
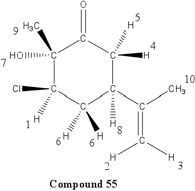
Place your mouse over the proton # to highlight the corresponding peak and H on the molecule.
| Proton # | Chemical shift (ppm) | Integration | Multiplicity | Explanation |
|---|---|---|---|---|
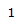 | 4.82 | 1H | t, J = 1.6 Hz | Has two 3-bond neighboring protons. Heavily deshielded by neighboring chlorine atom. |
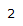 | 4.26 | 1H | t, J = 3.0 Hz | Part of an alkene group. Deshielded by double bond. Exhibits long-range coupling due to proximity to other protons due to orientation. |
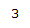 | 4.80 | 1H | s | Part of an alkene group. Deshielded by double bond. Besides 2-bond neighboring proton, is not oriented in close proximity to other protons. |
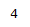 | 3.04 | 1H | t, J = 12.8 Hz | Part of sp3 carbon on ring. Exhibits coupling with 2-bond neighboring proton. Deshielded by carbonyl group. |
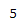 | 2.83 | 1H | tt, J = 12.7, 3.8 Hz | Part of sp3 carbon on ring. Exhibits coupling with 2-bond and 3-bond neighboring protons, as well as long range coupling. Deshielded by carbonyl group. |
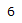 | 2.37-2.48 | 2H | m | Part of sp3 carbon on the ring. Exhibits complex coupling with other proton in pair, as well as 3-bond neighboring carbons. Also appears to exhibit long-range coupling. Deshielded by proximity to a carbon bonded to a chlorine atom. |
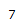 | 2.03 | 1H | s (broad) | Part of an –OH group. Is deshielded by oxygen atom. |
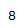 | 1.91 | 1H | dtd, J = 14.0, 3.6, 2.4 Hz | Part of a single-bonded carbon on the ring. Exhibits coupling with 3-bond neighboring protons, as well as long-range coupling. |
 | 1.76 | 3H | s | Part of a methyl group, and has no 3-bond neighboring protons. More deshielded than group 10 due to resonance from alkyne group. |
 | 1.67 | 3H | s | Part of a methyl group, and has no 3-bond neighboring protons. Slightly deshielded by carbonyl group. |
References
Richter, J.M.; Ishihara, Y.; Masuda, T.; Whitefield, B.W.; Llamas, T.; Pohjakallio, A.; Baran, P.S. J. Am. Chem. Soc. 2008, 130, 17938-17954.