Figure 1
Leading Question
Speculate as to why 55 was formed in a highly regio- and stereoselective manner.

Figure 1
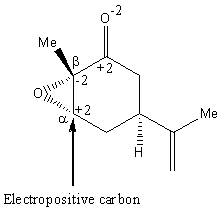
As epoxide rings have high strain, attack by a nucleophile at one of the epoxide carbons occurs preferentially to attack at the carbonyl carbon. Due to the electronegativity of the oxygen in the carbonyl group, the α-carbon is highly electropositive (Figure 1). Therefore, since TiCl4 is a Lewis acid that contains electronegative chlorine atoms, a chlorine atom from TiCl4 will prefer to attack at the α-carbon. In contrast, the β-carbon is highly electronegative and therefore is unfavorable to attack by a nucleophile.
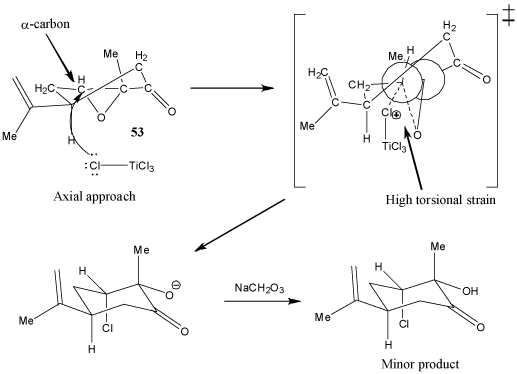
Figure 2
In addition, due to the fact that the epoxide group causes the ring to be highly conformationally constrained, the transition state resulting from axial attack of a chlorine atom in TiCl4 has a very high torsional strain (Figure 2). This torsional strain is much higher than the steric strain in the transition state resulting from equitorial attack of the chlorine atom (Figure 3). Therefore, equitorial attack of the chlorine atom in TiCl4 is highly prefered. As a result, the major product has the chlorine at the α-carbon in an equitorial position.
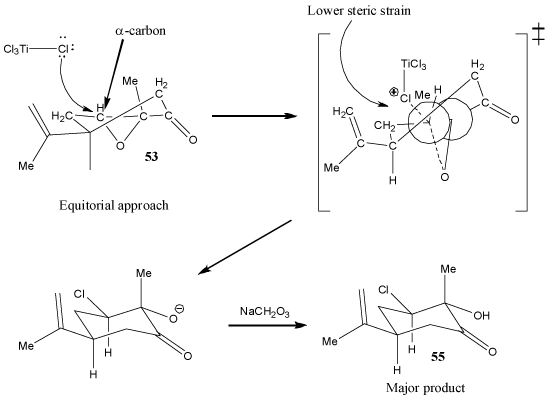
Figure 3
References:
Chérest, M.; Felkin, H. Tetrahedron Lett. 1968, 9, 2205-2208.
Richter, J.M.; Ishihara, Y.; Masuda, T.; Whitefield, B.W.; Llamas, T.; Pohjakallio, A.; Baran, P.S. J. Am. Chem. Soc. 2008, 130, 17938-17954.