|

NMR

HNMR Correlation of 15
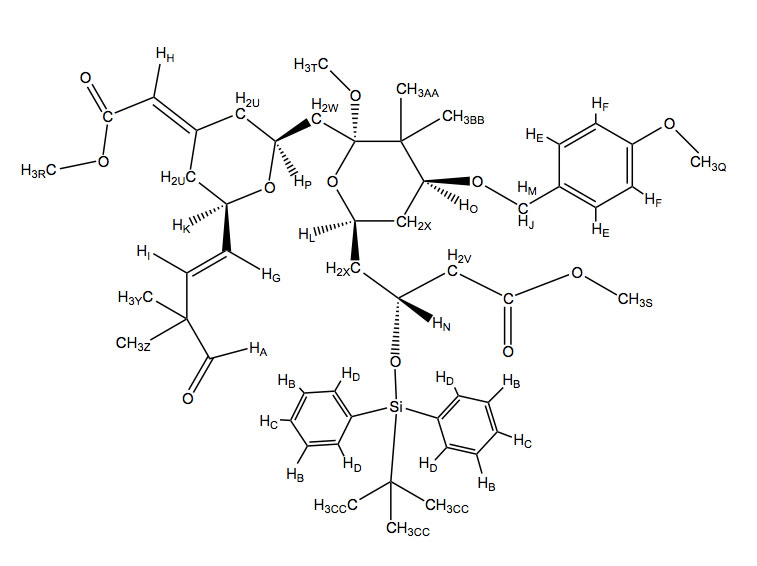
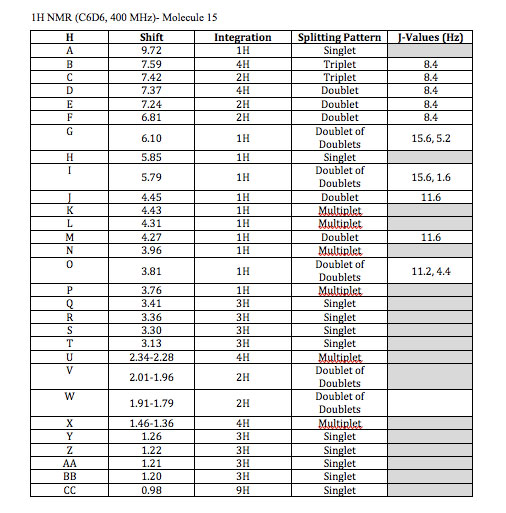
A. 9.72 (s, 1H)- An aldehyde hydrogen has a large chemical shift around 9.5-10.5, also it is a singlet because there are no 2 or 3 bond neighbors.
B. 7.59 (t, 4H)- Aromatic hydrogens fail in this range for chemical shifts. The silicon molecule is slightly electron donating which accounts for these 4 hydrogen atoms being more downfield due to their meta position. Also, there are two 3-bond neighbors that are both exactly 8.4 Hz away accounting for the triplet.
C. 7.42 (t, 2H)- These aromatic hydrogens are slightly higher field than expected due to the silicon atom and their relative para position. They have two 3-bond neighbors that are both exactly 8.4 Hz away accounting for the triplet.
D. 7.37(d, 4H)- These aromatic hydrogens are higher field than expected due to the electron donating capability of the silicon atom and the hydrogens relative ortho position.
E. 7.24 (d, J = 8.4 Hz, 2H)- This chemical shift again refers to aromatic hydrogens. The electron donating methoxy group shifts these meta hydrogens more downfield. They both have one 3-bond neighbor as well.
F. 6.81 (d, J = 8.4 Hz, 2H)- The chemical shift refers to a pair of aromatic hydrogens, These hydrogens are affected by the electron donating methoxy group shifting them higher field due to their ortho position. They also both have one 3-bond neighbor
G. 6.10 (dd, J = 5.2, 15.6 Hz, 1H)- This shift refers to alkene hydrogens. When determining which hydrogen it is you notice that it is a trans (15.6 Hz) doublet of doublet which can only occur at one of two locations. The other spin coupling is 5.2 Hz which could be a 3-bond neighbor which eliminates the other hydrogen because it doesn’t have two three bond couplings.
H. 5.85 (s, 1H-) This shift refers to alkene hydrogens. There is only one hydrogen that does not couple with other hydrogens and this is the singlet.
I. 5.79 (dd, J = 1.6, 15.6 Hz, 1H)- This shift refers to alkene hydrogens. When determining which hydrogen it is you can look at the 1.6 Hz and 15.6 Hz coupling. The 15.6 Hz coupling must refer to trans stereochemistry and the 1.6 Hz coupling would be a small 4-bond coupling. As this is the last alkene proton left to assign it is clear that this explanation fits its assignment.
J, M. 4.45 (d, J = 11.6 Hz, 1H) , 4.27 (d, J = 11.6 Hz, 1H)- These two hydrogens are shifted downfield because of their location next to an oxygen atom and an aromatic ring. It would be impossible to differentiate which hydrogen is which from the data given but it is clear that they couple with each other because both are doublets with a coupling constant of 11.6 Hz.
K. 4.43 (m, 1H)- This hydrogen is connected to an electronegative oxygen atom and an sp2 carbon, this accounts for its chemical shift. It would also be a multiplet because of its coupling with three different 3-bond neighbors.
L. 4.31 (m, 1H)- This hydrogen is connected to an electronegative oxygen atom and sp3 carbons accounting for its chemical shift. It would also be a multiplet because of its coupling with four different 3-bond neighbors.
N. 3.96 (m, 1H)- This hydrogen is connected to an electronegative oxygen atom and sp3 carbons accounting for its chemical shift. It would also be a multiplet because of its coupling with four different 3-bond neighbors.
O. 3.81 (dd, J = 4.4, 11.2 Hz, 1H)- This hydrogen is connected to an electronegative oxygen atom and sp3 carbons accounting for its chemical shift. It would also be a doublet of doublet because it couples with two different hydrogen atoms.
P. 3.76 (m, 1H)- This hydrogen is connected to an electronegative oxygen atom and sp3 carbons accounting for its chemical shift. It would also be a multiplet because it couples with fourth different hydrogen atoms.
Q, R, S, T. 3.41 (s, 3H), 3.36 (s, 3H), 3.30 (s, 3H) 3.13 (s, 3H)- All of these four, three-hydrogen singlets, refer to the hydrogens on the four methoxy groups. Distinguishing between these four would be difficult. However we can estimate that the order from most to least downfield would be the aromatic methoxy, the ester near an alkene, the ester, then the methoxy connected to the sp3 carbon respectively due to resonance contributors.
U. 2.34-2.28 (m, 4H)- These 4 hydrogens are next to an sp2 carbon. They are all diastereotopic to eachother and it would be impossible to tell the exact chemical shift for each with the information given because they are so similar. They also have multiplet coupling patterns.
V. 2.01-1.96 (dd, 2H) – These hydrogens are connected to a carbon connected to another carbon with an ester group and another with an electronegative oxygen attached to it. This slightly shifts the hydrogens more downfield. They both also couple with each other and one other hydrogen.
W. 1.91-1.79 (dd, 2H)- These hydrogens are connected to a carbon connected to two other carbons with electronegative oxygen atoms attached to them. This slightly shifts the hydrogens more downfield. They both also couple with each other and one other hydrogen.
X. 1.46-1.36 (m, 4H)- These hydrogens are connected to a carbon connected to another carbon with two electronegative oxygen atom attached to it. This slightly shifts the hydrogens more downfield. They both have a complex splitting pattern and couple with multiple different hydrogens.
Y, Z, AA, BB, CC. 1.26 (s, 3H), 1.22 (s, 3H), 1.21 (s, 3H), 1.20 (s, 3H), 0.98 (s, 9H)- These last peaks refer to hydrogen atoms on a methyl group. The way to distinguish between them is through careful analysis of the chemical shifts. For (Y), (Z), (AA), and (BB) it would be impossible to distinguish between (Y) and (Z) and also impossible to distinguish between (AA) and (BB) with the information given. However, looking at the position of the alkene and aldehyde group relative to (Y) and (Z) it is clear that these are slightly more downfield than the other methyl groups, so they earn the 1.26 and 1.22 ppm shifts. This means that (AA) and (BB) would correspond to the 1.20 and 1.21 chemical shifts. Clearly (CC) refers to the 0.98 ppm shift because of its nine equivalent hydrogens.
HNMR Correlation of 3
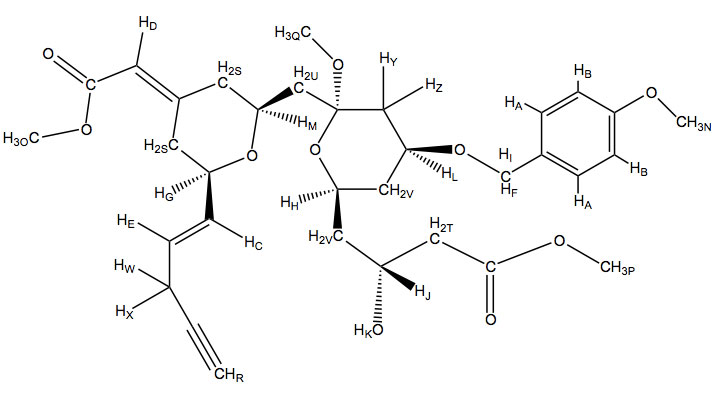
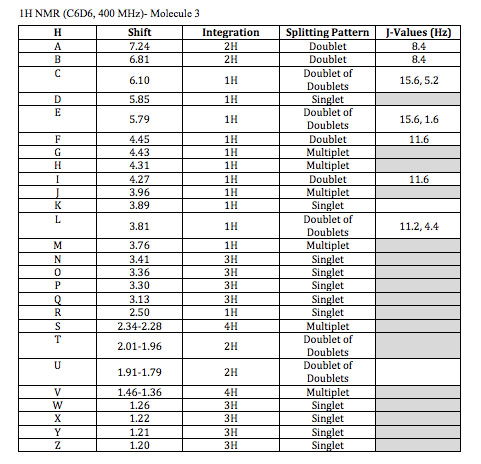
A. 7.24 (d, J = 8.4 Hz, 2H)- This chemical shift again refers to aromatic hydrogens. The electron donating methoxy group shifts these meta hydrogens more downfield. They both have one 3-bond neighbor as well.
B. 6.81 (d, J = 8.4 Hz, 2H)- The chemical shift refers to a pair of aromatic hydrogens, These hydrogens are affected by the electron donating methoxy group shifting them higher field due to their ortho position. They also both have one 3-bond neighbor
C. 6.10 (dd, J = 5.2, 15.6 Hz, 1H)- This shift refers to alkene hydrogens. When determining which hydrogen it is you notice that it is a trans (15.6 Hz) doublet of doublet which can only occur at one of two locations. The other spin coupling is 5.2 Hz which could be a 3-bond neighbor which eliminates the other hydrogen because it doesn’t have two three bond couplings.
D. 5.85 (s, 1H-) This shift refers to alkene hydrogens. There is only one hydrogen that does not couple with other hydrogens and this is the singlet.
E. 5.79 (dd, J = 1.6, 15.6 Hz, 1H)- This shift refers to alkene hydrogens. When determining which hydrogen it is you can look at the 1.6 Hz and 15.6 Hz coupling. The 15.6 Hz coupling must refer to trans stereochemistry and the 1.6 Hz coupling would be a small 4-bond coupling. As this is the last alkene proton left to assign it is clear that this explanation fits its assignment.
F, I. 4.45 (d, J = 11.6 Hz, 1H) , 4.27 (d, J = 11.6 Hz, 1H)- These two hydrogens are shifted downfield because of their location next to an oxygen atom and an aromatic ring. It would be impossible to differentiate which hydrogen is which from the data given but it is clear that they couple with each other because both are doublets with a coupling constant of 11.6 Hz.
G. 4.43 (m, 1H)- This hydrogen is connected to an electronegative oxygen atom and an sp2 carbon, this accounts for its chemical shift. It would also be a multiplet because of its coupling with three different 3-bond neighbors.
H. 4.31 (m, 1H)- This hydrogen is connected to an electronegative oxygen atom and sp3 carbons accounting for its chemical shift. It would also be a multiplet because of its coupling with four different 3-bond neighbors.
J. 3.96 (m, 1H)- This hydrogen is connected to an electronegative oxygen atom and sp3 carbons accounting for its chemical shift. It would also be a multiplet because of its coupling with four different 3-bond neighbors.
K. 3.89 (s, 1H)- This one hydrogen singlet does not couple with anything and has a chemical shift of 3.89. An OH group would fit these criteria and fortunately there is one in this molecule.
L. 3.81 (dd, J = 4.4, 11.2 Hz, 1H)- This hydrogen is connected to an electronegative oxygen atom and sp3 carbons accounting for its chemical shift. It would also be a doublet of doublet because it couples with two different hydrogen atoms.
M. 3.76 (m, 1H)- This hydrogen is connected to an electronegative oxygen atom and sp3 carbons accounting for its chemical shift. It would also be a multiplet because it couples with fourth different hydrogen atoms.
N, O, P, Q. 3.41 (s, 3H), 3.36 (s, 3H), 3.30 (s, 3H) 3.13 (s, 3H)- All of these four, three-hydrogen singlets, refer to the hydrogens on the four methoxy groups. Distinguishing between these four would be difficult. However we can estimate that the order from most to least downfield would be the aromatic methoxy, the ester near an alkene, the ester, then the methoxy connected to the sp3 carbon respectively due to resonance contributors.
R. 2.50 (s, 1H) This single hydrogen has no coupling. This leads us to conclude that it is the alkyne hydrogen. The chemical shift of 2.5 ppm falls in the range of alkyne hydrogens as well.
S. 2.34-2.28 (m, 4H)- These 4 hydrogens are next to an sp2 carbon. They are all diastereotopic to eachother and it would be impossible to tell the exact chemical shift for each with the information given because they are so similar. They also have multiplet coupling patterns.
T. 2.01-1.96 (dd, 2H) – These hydrogens are connected to a carbon connected to another carbon with an ester group and another with an electronegative oxygen attached to it. This slightly shifts the hydrogens more downfield. They both also couple with each other and one other hydrogen.
U. 1.91-1.79 (dd, 2H)- These hydrogens are connected to a carbon connected to two other carbons with electronegative oxygen atoms attached to them. This slightly shifts the hydrogens more downfield. They both also couple with each other and one other hydrogen.
V. 1.46-1.36 (m, 4H)- These hydrogens are connected to a carbon connected to another carbon with two electronegative oxygen atom attached to it. This slightly shifts the hydrogens more downfield. They both have a complex splitting pattern and couple with multiple different hydrogens.
W, X, Y, Z. 1.26 (s, 3H), 1.22 (s, 3H), 1.21 (s, 3H), 1.20 (s, 3H)- These last peaks refer to hydrogen atoms on a methyl group. The way to distinguish between them is through careful analysis of the chemical shifts. For (W), (X), (Y), and (Z) it would be impossible to distinguish between (W) and (X) and also impossible to distinguish between (Y) and (Z) with the information given. However, looking at the position of the alkene and aldehyde group relative to (W) and (X) it is clear that these are slightly more downfield than the other methyl groups, so they earn the 1.26 and 1.22 ppm shifts. This means that (Y) and (Z) would correspond to the 1.20 and 1.21 chemical shifts.
|