Related References
Main Reference: Lemieux, R. U.; Ratcliffe, R. M. Can. J. Chem. 1979, 57, 1244-1251. See paper
Reference 1: Ferrari, B.; Pavia, A. A. Carbohydr. Res. 1980, 79, C1-C7. See paper
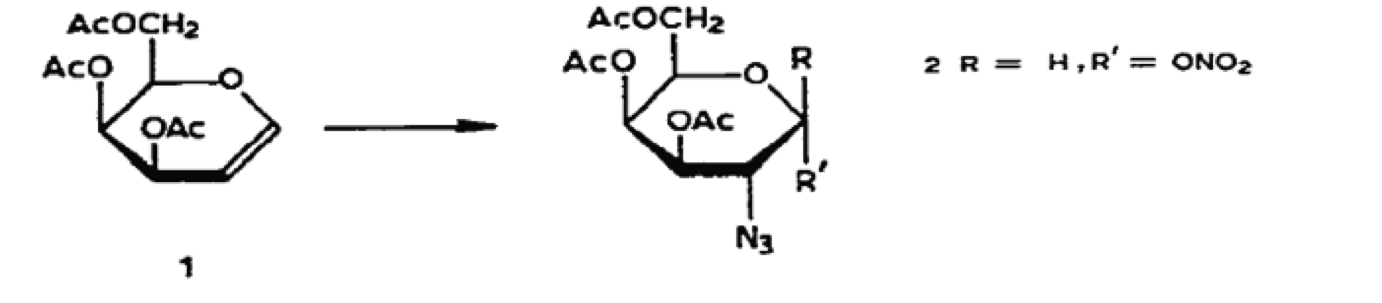
This research article concerns the synthesis of different types of glycopeptides. These molecules have a significant impact in biochemistry and include compounds such as blood-group substances and immunoglobulins. The reaction discussed in our article (azidonitration) provides the first key intermediate in the synthesis. Azide and nitrate groups are added across a cyclohexene double bond in 60% yield, and the stereoisomeric molecules were separated using column chromatography. The experimenters used the procedure described in our citation (Lemieux and Ratcliffe, 1979). The newly added nitrate is subsequently used as a leaving group and substituted with an iodide atom, and then replaced by a chloride ion. This works because the nitrate ion is quite stable; the pKa of nitric acid is -1.3, which speaks to the stability of the conjugate base. This final molecule serves as a useful intermediate for glycopeptide formation, and was used to subsequently create a number of different final products.
Reference 2: Tatsuta, K.; Akimoto, K.; Takahashi, H.; Hamatsu, T.; Annaka, M.; Kinoshita, M. Bull. Chem. Soc. Jpn. 1984, 57, 529-538. See paper
This research article concerns the synthesis of apramycin and saccharocin, two aminoglycoside antibiotics. Similar to Reference 1, the nitrate that is added is used as a leaving group. In this case, it is replaced by a methoxy group by means of a barium hydroxide catalyst. Eventually, the researchers successfully created derivations of the antibiotics and proved their inhibitory effect on bacterial growth.
Reference 3: Gambert, U.; Gonzalez Lio, R.; Farkas, E.; Thiem, J.; Verez Bencomo, V.; Lipták, A. Bioorg. Med. Chem. 1997, 5, 1285-1291. See paper
This research article concerns the synthesis of a specific oligosaccharide antigen, using an enzyme found in bovine testes. These types of molecules are key component of a wide variety of biological functions, so a cheap, efficient way to produce them is very desirable. Again, azidonitration is used to provide nitrate as a good leaving group. In this reaction, the azide group is also eventually replaced by means of a palladium catalyst.
Other References:
Mechanism: Lindhorst, T. K. Essentials of Carbohydrate Chemistry and Biochemistry, 3rd Ed. Weinheim, Germany: Wiley-VCH, 2007; p. 133
NMR Data:
Pragani, R.; Stallforth, P.; Seeberger, P. H.
Org. Lett. 2010, 12,
1624–1627.
