You need to make sure you understand the experimental for this mechanism. It's one of the most important aspects of the class -- application! The lab for this course is worth 2 credits, and if you don't do well, you won't make it out of college! Study hard!
Compounds 25/26 to 27
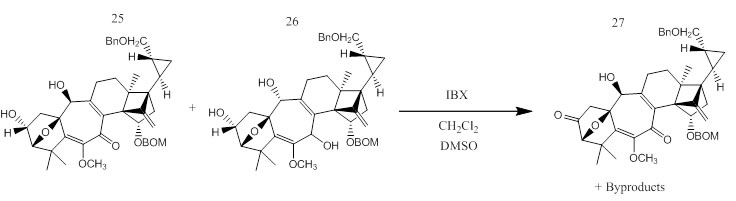
Molecules 25 and 26 were obtained from a mixture of molecule 24 (a triketone) and .89 mL of tulene. This mixture was added to a hexane solution of DIBAH (.32mL). The mixture was stirred and a Rochelle salt was added. The mixture was then seperated and dried over MgSO4 to result in molecules 25 and 26.
0.9mL of a crude mixture of 25 and 26 dissolved in DMSO-CH2Cl2 (1:2). 37.4mg (0.13mmol) of 2-iodoxybenzoic acid (IBX) was then added at room temperature, and the mixture was stirred for 1h. An aqueous NaHCO3 solution was added, and the mixture was separated. The aqueous layer was extracted with ether. The combined organic layer was dried over anhydrous MgSO4 and concentrated under reduced pressure to remove solvent. Silica gel column chromatography in EtOAc/hexane (1:7.3 then 1:4) was used for purification. 13.1mg (18.9µmol) of alcohol 27 (43% for 2 steps) was obtained as a pale yellow amorphous solid along with 16.9mg of a mixture of byproducts. The byproducts were then oxidized with DMP to give the original starting material, molecule 24.
