Glossary
Just like the spinner in the board game, the glossary is something that will "move you along" the class. Without understanding these key words, you won't be able to understand the mechanism or experiment! The spinner thus the glossary are essential.
----------------------------------------------------------------------------------
Rochelle Salt
Solid Potassium sodium tartrate (KNaC4H4O6·4H2) is used is used in reactions to break up emulsions and is also used in the reagent that is used to measure protein concentrations.. It is also used in the manufacture of baking powder and is used sometime as a mild laxative. Rochelle salt has a large piezoelectric effect. This means that it has an electric charge on its surfaced induced by pressure, twisting, or bending. The piezoelectric effect of Rochelle salt was first discovered in 1880 by Pierre and Paul-Jacques Curie.

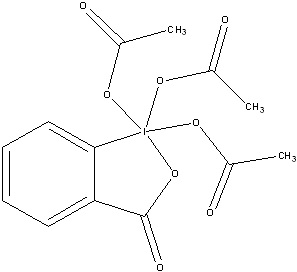
DMP
DMP is an abbreviation for Dess-Martin periodinane. DMP is a reagent that is commonly used to oxidize primary alcohols to aldehydes as well as to oxidize secondary alcohols to ketones. DMP is synthesized from another oxidizing agent: 2-Iodoxybeonzoic Acid. DMP was first synthesized by Daniel Benjamin Dess and James Cullen Martin in 1983. The benefit of DMP over IBX is that it is much more soluble in many organic solvents.
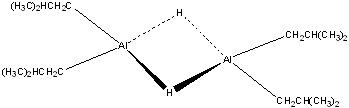
DIBAH
Diisobutylaluminum hydride (DIBAH), is a reducing agent that is used in organic syntesis to reduce esters and nitriles to aldehydes. The problem with DIBAH is that it violently reacts with air and water and can sometime cause fires. Unlike another common reducing agent, LiAlH4, DIBAh reactss more quickly with electron-rich compounds and therefore is considered to be an electrophilic reducing agent (rather than a nucleophilic reducing agent).
Sources
Rochelle Salt. Photograph. Seawhy.com.
"Dess-Martin Periodinane." Organic-chemistry.org. Organic Chemistry Portal. Web. 04 Apr. 2012.
"Diisobutylaluminum Hydride." Science.uvu.edu. Utah Valley University. Web. 04 Apr. 2012.
