EXPLANATION
HYDROGEN
SHIFT & MULTIPLICITY
Hydrogen A has two vicinal hydrogen neighbors on the cyclobutane ring; the doublet of doublet ostensibly overlaps to appear as a triplet. Coupling constant of 9.7 Hz shared by Hydrogen D.
A
3.15 (triplet)

B
3.18 (triplet)
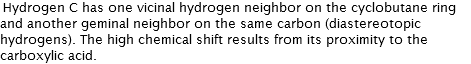

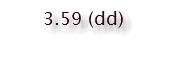
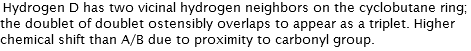

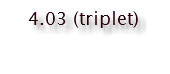


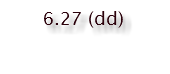







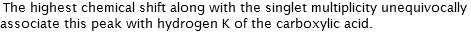

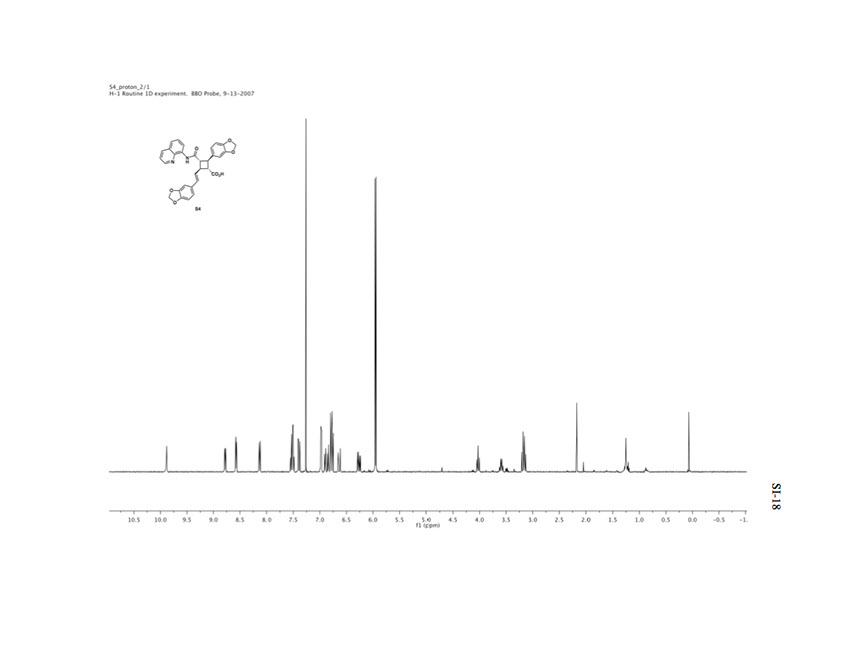

Protip: Mouseover any labeled hydrogen on molecule 18 to see the corresponding peak on the proton-coupled H-NMR spectrum.
Poor resolution due to low quality spectrum in the supplemental information.
University of Michigan Chem 215/216 HH Winter 2014. Nicholas Carducci's Structured Study Group. HTML Project of Callie Chappell, James Lawniczak, Aiman Faruqi, and Ryan Gentil
