|
Mulan finds Little Brother on the floor, bored out of his mind. She has a plan to keep him busy! But first, she has an interesting article for him to read.... |
|
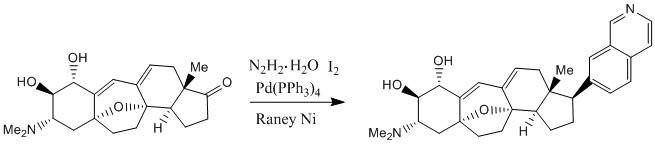
| Science Citation Index: Article Cited by the Main Article Shi, J.; Shigehisa, H.; Guerrero, C. A.; Shenvi, R. A.; Li, C.-C.; Baran, P. S. Angew. Chem. Int. Ed. (English) 2009, 48, 4328-4331. |
||
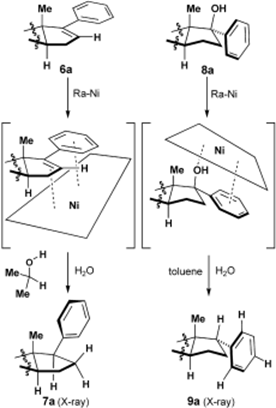
| In this article, the authors studied two ways of using Raney’s nickel to selectively choose the stereochemistry of a phenyl group attached to the D ring of aryl steroids. As can be seen in Figure 1, when Raney nickel reduced a double bond connected to a phenyl group, the phenyl group ended up exclusively cis to the methyl group located on the same ring. However, when Raney nickel was used to remove an alcohol group cis to the methyl, the phenyl group ended up exclusively trans to the methyl. By using deuterium experiments, the researchers established that the hydrogens used to hydrogenate the double bond come from Raney nickel, not from one of the solvents. The authors propose that Raney nickel is able to so selectively determine the stereochemistry of the phenyl group because of the facial selectivity of the aryl steroid. As seen in Figure 2, the double bond cannot be reduced to make the phenyl group trans to the methyl because the methyl prevents bonding between the flat metal face and the double bond from this orientation. | Figure 1 | Figure 2 |
 |
 |
|

Mulan cannot fully explain her mechanism with only one source.
Can our Little Brother help Mulan?
First, the Little Brother chases after the bone with a seed bag attached to him. |
Chen, D. Y.-K.; Tseng, C.-C. Org. Biomol. Chem. 2010, 8, 2900-2911. This article describes several reports of the total synthesis of Cortistatin A, a compound that is currently being investigated for its anti-angiogenic agents. In the first completed synthesis of a molecule in the cortistatin family, Baran and colleagues started with prednisone and through a series of innovative steps arrived at Cortistatin A. This reaction pathway is very commercially feasible because it utilizes a starting material that is both feasible economically and readily accessible. Raney Nickel is used as a reducing agent in the last step of the reaction to provide a chemo- and stereoselective hydrogenation of one double bond.
|
Next, the Little Brother then runs to the garden to feed chickens. |
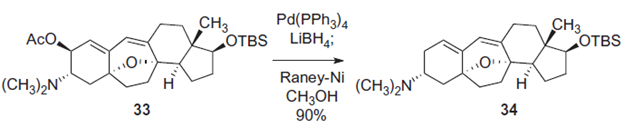
Flyer, A.; Si, C.; Myers, A. Nat. Chem. 2010, 2, 886-892. This article describes different approaches to synthesize the cortistatin family, cortistatin A, J, K, and L. The compounds within the family vary slightly by substituents on the A- and D-rings of the main steroidal skeleton. The author and colleagues specifically look to prepare the compounds from the single azido alcohol intermediate. While the synthesis of cortistatin K is more direct than cortistatin A, the author used Raney-Nickel on cortistatin K to reduce the C=O bond and add hydrogens. First, lithium borohydride and tetrakis(triphenylphosphine)-palladium regioselectively did reductive cleavage of the allylic C-O bond. Then, Raney nickel in methanol achieved decomplexation, or deformation of complex bonds. In this case, the decomplexation would be treated on the allylic C-O bond. Raney nickel here completed the bond reduction of cleaving C-O and forming C-H instead.
|
Then, the Little Brother passes in front of Mulan's horse. |
Naragan, A. R. H.; Simmons, E. M.; Sarpong, R. Eur. J. Org. Chem. 2010, 19, 3553-3562. This article is an organized compilation of most of the research done to synthesize cortistatin A. There is a semi-synthesis, three total syntheses, and two formal syntheses. Some research teams have tried a ring-expansion approach, which required careful calculation and stereoselective reactions. There is also the oxidizing approach, where an aromatic ring in particular is oxidized in the process to create cortistatin A. It is mostly helpful in total synthesis and helped create the ether in the molecule. Pericyclic transformations, electrocyclization, cycloaddition, and domino-sequences were used to explore different syntheses, but the synthesis that was most relevant to our paper was one using Raney nickel to do a dehydration reaction and leave another hydrogen in its place. This would maintain the stereochemistry that was created earlier in the experiment, which is similar to our paper, since the stereocenter in our molecule was created because Raney nickel reacts on one side of the molecule.
|

In the end, the Little Brother successfully finishes helping Mulan and gets the most delicious reward.
 Need more help?
Need more help?
Works Cited
Chen, D. Y.-K.; Tseng, C.-C. Org. Biomol. Chem. 2010, 8, 2900-2911.
Flyer, A.; Si, C.; Myers, A. Nat. Chem. 2010, 2, 886-892.
High Performance Liquid Chromatography - HPLC. http://www.chemguide.co.uk/analysis/chromatography/hplc.html (accessed Mar 23, 2014).
Life Sciences Mass Spectrometry Facility, University of Bristol. High Performance Liquid Chromatography Mass Spectrometry (HPLC/MS). http://www.bris.ac.uk/nerclsmsf/techniques/hplcms.html (accessed Mar 23, 2014).
Master Organic Chemistry. Reagent Friday: Raney Nickel. http://www.masterorganicchemistry.com/2011/09/30/reagent-friday-raney-nickel/ (accessed Mar 23, 2014).
Naragan, A. R. H.; Simmons, E. M.; Sarpong, R. Eur. J. Org. Chem. 2010, 19, 3553-3562.
Paul’s Lab: Raney Nickel. http://www.paulslab.com/chemicals/rochelle-salt.html (accessed Mar 23, 2014).
Shi, J.; Monolikakes, G.; Yeh, C.-H.; Guerrero, C. A.; Shenvi, R. A.; Shigehisa, H.; Baran, P. S. J. Am. Chem. Soc. 2011, 133, 8014-8027.
Shi, J.; Shigehisa, H.; Guerrero, C. A.; Shenvi, R. A.; Li, C.-C.; Baran, P. S. Angew. Chem. Int. Ed. (English) 2009, 48, 4328-4331.
UCDavis ChemWiki. Organic Chemistry: Catalytic Hydrogenation of Alkenes. http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkenes/Reactions_of_Alkenes/Catalytic_Hydrogenation (accessed Mar 23, 2014).
Wikipedia. Cortistatins. http://en.wikipedia.org/wiki/Cortistatins (accessed Mar 23, 2014).