Crooner Curve
Crooner Curve
Every accomplished driver must not only know his car inside and out, but each part of the race track must be understood as well. Thus, it is absolutely imperative to understand the mechanics behind the reaction at each step in the mechanism. At the Crooner Curve, we observe the first part of the Boc deprotection in the molecule.

The starting molecule for this reaction contains two Boc protection groups. The first step in the mechanism is to deprotect the molecule with an acid, trifluoroacetic acid (TFA).
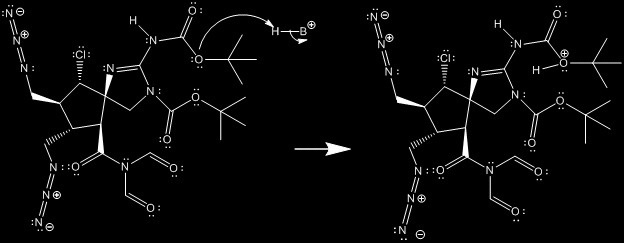
The mechanism begins with an acid catalyzed protonation of the oxygen attached to the tert-butyl group of the Boc protecting group on the secondary amine. Subsequently, the carbon oxygen bond between the tert-butyl and the oxygen will cleave heterolytically, causing a tertbutyl cation to leave.

The conjugate base of the acid deprotonates the hydroxy group of the protecting group and pushes the electrons such that another equivalent of acid protonates the nitrogen on which the Boc group was attached. This will cause the rest of the Boc group, originally a COOH, to leave as carbon dioxide.
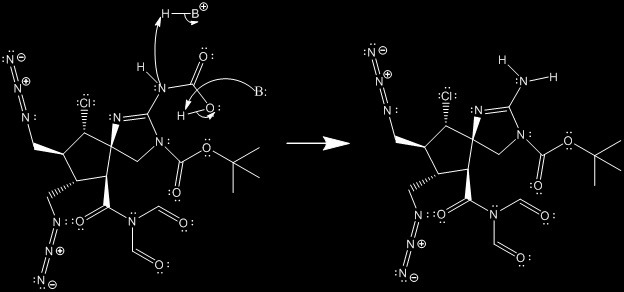
In the next reaction we view a similar mechanism that begins to deprotect the amine in the ring. We can start seeing this reaction in the next turn... the Wilson.