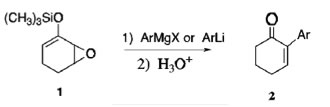Original ArticleRichter, J. M.; Ishihara, Y.; Masuda, T.; Whitefield, B. W.; Llamas, T.; Pohjakallio, A.; Baran, P. S. J. Am. Chem. Soc. 2008, 130, 17938-17954. |
||
Source 54 within original article: Wender, P. A.; Erhardt, J. M.; Letendre, J. L. J. Am. Chem. Soc. 1981, 103, 2114-2116. |
||
First source citing Wender: Baran, P. S.; Richter, J. M. J. Am. Chem. Soc. 2005, 127, 15394-15396. If we were to compare scheme one of this article to scheme 10 of our original article, we would see that both articles use Wender’s study in support of their data. We specifically see same Cl replacement of the OH group and the same general chemistry in steps 6 to 7 of this article. Both our original article and this article discuss the SN2 pathways of enolate-bearing carbons and use the same mechanisms.
|
||
Second source citing Wender: Kozmin, S. A.; Iwama, T.; Huang, Y.; Rawal, V. H. J. Am. Chem. Soc. 2002, 124, 4628-4641. As was discussed in our original article, we see the use of cyclohexenones. The difference is that in the original article, enolates were used, but the general chemistry of the mechanism is quite similar. Both this article and our original article involve an SN2 substitution with the use of strong bases. |
||
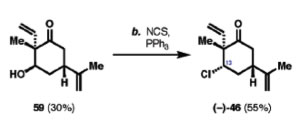
Third source citing Wender: Majetich, G.; Liu, S.; Fang, J.; Siesel, D.; Zhang, Y. J. Org. Chem. 1997, 62, 6928-6951. As seen in the second source, this source discusses the sequence for the preparation of dienones by opening the epoxide ring with the process of oxidation. Wender’s techniques and laboratory data was applied to this article. Finally, the step shown in this article of the ring opening can be compared to the step of our original article in the preparation of compound 59.
|