


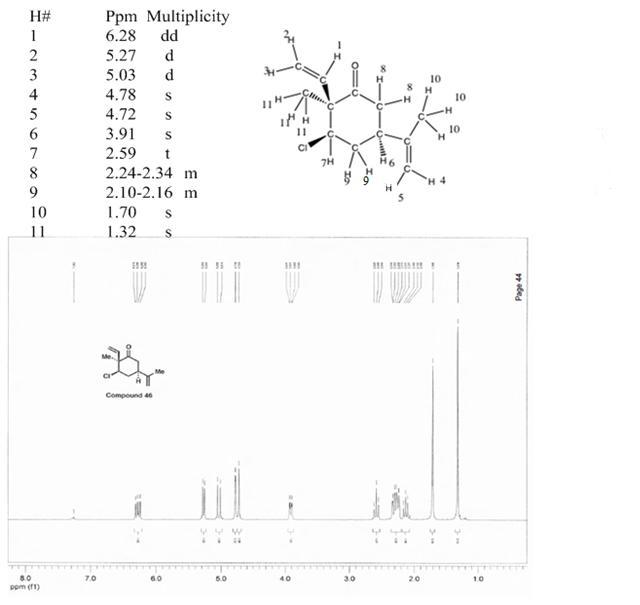
The most down-field shifted hydrogen is that of an Sp2 Carbon that is at a beta position with respect to the ketone group (6.28ppm). Hydrogens #2 and 3 are also sp2 carbons, but are
further away from the electronegative Carbonyl group (5.27, 5.03ppm). #4 and 5 are shifted more upfield from #3 and 4 because they are further away from the carbonyl group. The rest
of the sp3 hydrogens were systematically assigned based on integration (eg methyls have an integration of 3) and by chemical shifts as shown by the table above. Mouse over for correspondence.
.

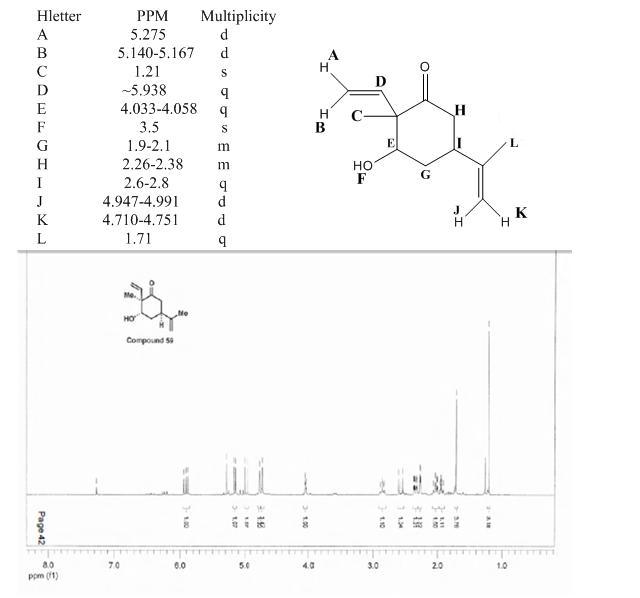
Hydrogens "A and B" are assigned downfield positions of (5.275 and 5.15ppm) because they are of an sp2 hybridization. Hydrogen D is more downfield shiftedbecause it is closer to the Carbonyl group
which is electron withdrawing. Hydrogens J and K are assigned more upfield shifts of the sp2 hydrogens due to their bond distance from electron withdrawing groups. Since Hydrogen F is the only singlet
shifted downfield enough, it must be the hydrogen of a hydroxyl group. Hydrogen E must be shifted downfield (4.0ppm) because it is attached to a carbon attached to an oxygen. Also, since there were only
two groups with an integration of three, hydrogens C and L must be methyl hydrogens. Since L is shifted more downfield, it must be the sp2 methyl group. The rest of the sp3 hydrogens were systematically
assigned based on integration, three bond couplings and chemical shifts. Mouse over for correspondence.
Now that you see,
what it means to be me,
...depleted of youth
Home | Mechanism | Experimental | Citations | Leading Question | About Us
>