Experimental for 21 to S1:
To a stirred solution of 21 (2.60 g, 4.35 mmol) in THF (22 ml) was added lithium aluminum hydride (496 mg, 13.0 mmol) at room temperature and the solution
was stirred for 1 h at 70 ºC. After dilution with diethyl ether, the reaction mixture was
quenched with excess amount of aqueous sodium hydroxide. The resulting white
precipitate was removed by filtration.
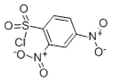
To the filtrate was added 2,4-dinitrobenzenesulfonyl chloride (1.16 g, 4.35 mmol), and the solution was stirred
for 10 min at room temperature. The mixture was extracted with ethyl acetate, washed
with water, dried over sodium sulfate, and filtered. Concentration of the filtrate gave a
crude sulfonamide, which was used for the next step without further purification.
To a stirred solution of the crude sulfonamide in methanol (22 ml) was added
camphorsulfonic acid (101 mg, 0.44 mmol) at room temperature. After stirring at room
temperature for 6 h, aqueous sodium bicarbonate was added, and the solution was
extracted with dichloromethane. The combined organic solution was washed with brine, dried over sodium sulfate, and filtered. The filtrate was concentrated in vacuo and the residue was purified with flash column chromatography (50% ethyl acetate in n-hexane) to alcohol S1 (1.75 g, 68% for 2 steps) as a yellow oil.
[α]D 24 –3.18º (c 1.00, CHCl3); IR (film) 3490, 2937, 1554, 1505, 1353, 1275, 1119 cm-1; 1H NMR (400 MHz, CDCl3) δ 8.46 (s, 1 H), 8.45 (d, J = 8.5 Hz, 1 H), 8.07 (d, J = 8.5 Hz, 1 H), 6.78 (d, J = 8.4 Hz, 1 H), 6.75 (d, J = 8.4 Hz, 1 H), 6.02 (dd, J = 10.0, 1.6 Hz, 1 H), 5.83 (ddd, J = 10.0, 5.9, 2.3 Hz, 1 H), 4.53 (t, J = 4.5 Hz, 1 H), 4.37 (d, J = 8.5 Hz, 1 H), 4.37 (s, 3 H), 4.37 (1 H), 3.37 (s, 3 H), 3.35 (m, 1 H), 3.31 (s, 3 H), 2.97 (m, 1 H), 2.91 (s, 3 H), 2.86 (d, J = 4.5 Hz, 1 H), 2.38-2.45 (m, 1 H), 2.04-2.16 (m, 2 H), 1.93-2.00 (m, 1 H); 13C NMR (100 MHz, CDCl3) δ 149.6, 148.0, 145.3, 143.8, 137.8, 132.5, 131.6, 128.5, 126.0, 125.7, 125.4, 123.7, 119.7, 111.7, 105.1, 90.1, 67.6, 55.8, 54.2, 53.2, 51.5, 46.3, 37.4, 35.3, 34.9, 29.3; HRMS (ESI) calcd for C26H31N3O11SNa (M+Na)+ : 616.1577, found 616.1604.
Glossary:
2,4-dinitrobenzenesulfonyl chloride: yellow crystalline powder.
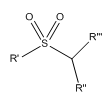
Sulfonamide: a functional group where a sufonyl group is attached to an amine group.
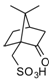
Camphorsulfonic acid (CSA): a relatively strong organosulfur acid. Colorless solid soluble in organic solvents.
In Vacuo: in vacuum, a space devoid of air.
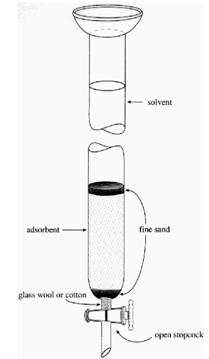
Flash Column Chromatography: A form of column chromatography that occurs much quicker due to the use of increased air pressure. The column is a long glass tube fitted with a stopcock and topped with a standard taper glass joint. To prepare, the column is dry packed with "Flash" silica gel to about 6"-10", then filled with solvent, which is forced through with air. Samples are collected at a flow rate of about 2in/min.
HRMS: (High resolution mass spectrometry) a technique very much like regular mass spectrometry, where molecules are weighed based upon the motion of charged particles, however HRMS is capable of calculating the exact mass of an ion.

