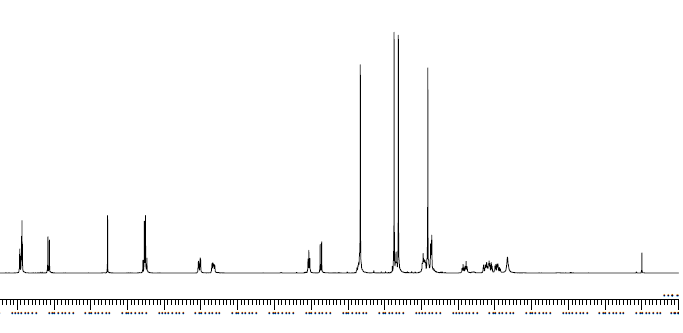
NMR for Molecule S1:
Explanations for S1:
a: This is an aromatic singlet, that would be very downfield due to electron withdrawing groups, so the only value to match would be 8.46.
b: This is another aromatic but with one neighbor, c, it is near an electron withdrawing group so it would be downfield, at around 8.45
c: Another aromatic doublet, but not as close to an ewg as the other two, so it would be a little more upfield, and couples with b.
d: Aromatic hydrogens show up near 7, this hydrogen is not as close to a large deshielding group so is would show up more downfield, but not by much.
e: Since e is aromatic and closer to a larger deshielding group, it would show up somewhat upfield.
f: This hydrogen has one neighbor and is attached to a double bond, so it would appear quite downfield. Since it is on a double bond, it would not share the share the exact same coupling constant as its neighbor so it would appear with more complex peaking—a dd.
g: This hydrogen has three neighbors and would normally show up around a quintet, but they do not share the same coupling values. So three neighbors would become a ddd, the only value to have this is 5.83, which also makes sense since it is a hydrogen on a double bond. We can also tell from the coupling value that it needs to be near whichever hydrogen had the value of 6.02.
h: A hydrogen close to two oxygens would be very downfield, and shows up at a triplet with only two neighbors, the only peak to match is 4.53.
i: This hydrogen only has one neighbor, so it would show up as a doublet. It's very close to oxygen as well as a carbon bonded to a hydroxyl group, so it would need to be pretty downfield.
j: This is a 3H singlet attached to an oxygen and should show up around 3.8, since it is attached to a benzene ring, we would expect it to be even more downfield at 4.37.
k: This would be very downfield because it is directly attached to a hydrogen so it was assigned 4.37, which is only labeled as 1H. This is a little confusing since it seems like it would have neighbors, however.
l: This is a 3H singlet attached to an oxygen and should show up around 3.8.
m: This hydrogen is attached to a ring and very close to a hydroxyl, it would be very downfield, it also has several neighbors that are coupled different, so it was assigned to 3.35 as a multiplet.
n: This is a 3H singlet attached to an oxygen and should show up around 3.8.
p: This is a 3H singlet attached to an nitrogen, so it would show up a little more upfield, near 3.
q: This hydrogen was labeled at 2.86 because it is near h and they both have J values of 4.5, so much participate in coupling.
s: These two hydrogens are sp3 and not extremely close to any electronegative atoms so they were assigned at 2.04-2.16.
As for the remaining multiplets, o, r, t assigning these were difficult. o was assigned the most downfield value because it is attached to a nitrogen, r is close to a deshielding group so it would be a little more upfield near 2-2.5, and the hydrogens assigned to t are the most like a normal sp3 group that is not near many deshielding groups, so it could be seen as the most upfield of all the values.