Before diving into the experimental procedure, a proper comprehension of the stereochemistry involved in the reaction is necessary! The answer lies just over Gumdrop Mountain!
Leading Question
Diastereoselectivity of the Cyclopropanation Reaction
Cyclopropane rings are 3 carbon rings with bond angles of roughly 60 degrees. They are naturally occurring biological subunits that have powerful activity, and are also useful intermediates in organic synthesis reactions. Many approaches have been used to accomplish cyclopropanation, most notably through zinc carbenoids reacting with alkenes. In our article, the authors used this method, along with a dioxaborolane ligand to obtain a diastereoselective (94:6) product.
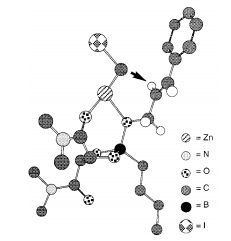
The dioxaborolane ligand was designed so that it contains amide groups that act as Lewis bases to chelate the ZnCH2I2 (cyclopropanating reagent) and a boron that acts as a Lewis acid and can bind to the oxygen atom of the alcohol. When the dioxaborolane ligand binds to the oxygen molecule, the Lewis base site on the ligand will complex with the ZnCH2I2 and hold it in position by the alkene. Since the alkene in our reaction was E, the hydrogen atoms are already trans to each other. When the reaction proceeds, the trans hydrogens are pushed anti to each other in the product. If the alkene was Z, then the syn product would have been diastereoselectively produced. The 3D transition state image below shows the cyclopropanation of cinnamyl alcohol, but the mechanism is exactly the same as the cyclopropanation of the alkene in alcohol S5.