| Home | Leading Question | Mechanism | Experimental | H-NMR Spectra | References | About Us |
|---|
Leading Question
Today we have a special interactive tutorial for all of you. Our leading question is as follows: develop a tutorial for your classmates on the Sharpless asymmetric epoxidation reaction.
The reactants that you need for this reaction are as follows:
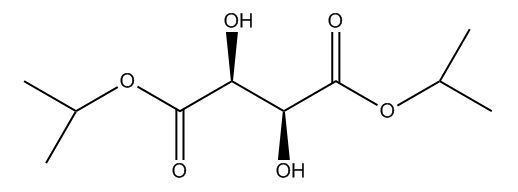
- Diisopropyl D-tartrate or (-)DiPT - used as a ligand to reform the titanium catalyst
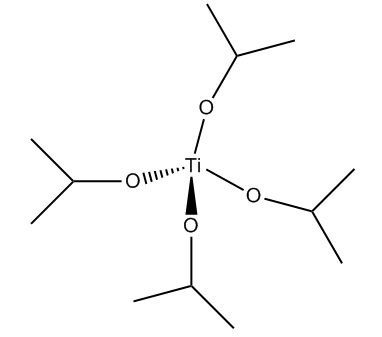
- Titanium isopropoxide or Ti(iPrO4) - used as a catalyst in order to form the epoxy group
- tert-Butyl hydroperoxide or tBuOOH - attaches to the titanium catalyst to act as a source of oxygen for the epoxy group

With these three reactants, you will be able to transfigure an allyl alcohol into a chiral group with a hydroxymethyl and an epoxy group! For more information about how the mechanism works, look at the second step of our mechanism on the "Mechanism" page. If you are more interested in the exact amounts of reagents and conditions for the reaction, please visit our "Experimental" section.
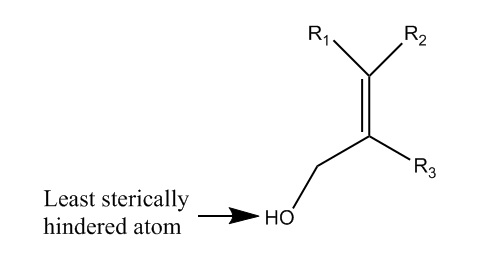
Now, we will explain some of the various characteristics of this reaction which make the Sharpless epoxidation an interesting and special transfiguration. For the second step, much like the first, the titanium catalyst can only bind to the molecule in one way. There are no other spots where the titanium could favorably attack the product of the first step. The favorability of the attack is mostly due to sterics. Since the titanium catalyst is so large, it can only attack the molecule at the alcohol substituent.
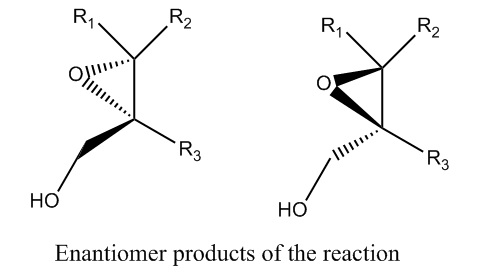
The only stereoisomers of the product for the second step would be enantiomers. The titanium catalyst could also produce a molecule with the oxygen going backwards instead of forwards. However, this should not matter because it is the enantiomer. Again, there is no favorable way to create a diastereomer, therefore the reaction is stereoselective as well as regioselective.
And there you have it, witches and wizards! You can now tell all of your fellow chemistry magicians how to transfigure an ordinary achiral molecule into a wonderful chiral molecule using the wonderful Sharpless epoxidation reaction.
Sources used:
Zheng, C.; Dubovyk, I.; Lazarski, K. E.; Thomson, R. J. J. Am. Chem. Soc. 2014, 136, 17750-17756.
Nagasawa, T.; Shimada, N.; Torihata, M.; Kuwahara, S. Tetrahedron 2010, 66, 4965−4969.