| Home | Leading Question | Mechanism | Experimental | H-NMR Spectra | References | About Us |
|---|
Experimental
Transfiguration is no easy magic. To transfigure effectively, one must follow an exact procedure with extreme precision and intense focus. Perhaps with enough practice and study, you will be able to master the following procedure and transfigure your chemicals correctly and efficiently.
Step 1: Sodium dodecyl sulfate (2.3 g, 8.0 mmol) and DMAP(9.78 g, 80 mmol) was added to a stirred suspension of Molecule 8 (10g, 80 mmol) in water (80 mL). The mixture was allowed to stir at room temperature for 15 minutes. Formaldehyde (37% in water, 80 mL) was added to the mixture and the mixture was allowed to stir for one hour at room temperature. The mixture was then quenched with brine and extracted three times with ethyl acetate. The combined organic layer was dried over anhydrous magnesium sulfate and concentrated. The residue was purified by silica gel chromatography using a ratio of 4:1 hexanes/ethyl acetate to give S2 (8.81 g, 57 mmol, 71%). 1 H NMR (400 MHz CDCl3) δ 6.58 (s, 1H), 4.20 (d, J = 6.5, 1.1 Hz, 2H), 2.50 - 2.47 (m, 3H), 1.86 (d, J = 0.8 Hz, 2 H), 1.18 (s, 6H).
Step 2: Titanium(IV) isopropoxide (16.6 mL, 56 mmol), (-)-diisopropyl D-tartrate (11.2 mL, 57.6 mmol), and anhydrous dichloromethane (130 mL) was added to a flame dried round bottomed flask with a stir bar. The mixture was cooled down to -20°C and S2 (5.92 g, 38.4 mmol) was added as a solution in anhydrous dichloromethane (30 mL). The new solution was stirred at -20°C for 30 minutes, then was cooled further to -30°C. Tert-Butyl hydroperoxide (~5.5 M solution in nonane, 20.9 mL, 115.2 mmol) was added dropwise to the solution. The solution was then allowed to heat to -20°C and stirred for 36 hours (where TLC analysis showed the reaction was complete). The mixture was quickly filtered through celite. The filtrate was cooled down to -20°C and then quenched with iron(II)sulfate heptahydrate (32 g) and 10% aq tartaric acid (200 mL). After allowing the mixture to warm up to room temperature, the layers were separated. The aqueous layer was extracted with dichloromethane three times. The organic layer was dried over anhydrous magnesium sulfate and concentrated. The residue was purified using silica gel chromatography using a 19:1 to 2:1 ratio of hexanes/ethyl acetate to give 9 (4.83 g, 28.4 mmol, 74%, 94% ee). IR (neat): 3424, 1701, 2960, 2872, 1090, 1028 cm-1. 1 H NMR (500 MHz, CDCl3) δ 3.87 (dd, J = 12.8, 6.7 Hz, 1H), 3.82 (dd, J = 12.8, 3.8 Hz, 1H), 3.22 (d, J = 1.3 Hz, 1H), 2.43 (ddd, J = 18.8, 6.3, 3.2 Hz, 1H), 2.30 (d, J = 6.7 Hz, 1H), 2.20 (ddd, J = 18.8, 11.6, 6.9 Hz, 1H), 1.87 (ddd, J = 13.6, 11.6, 6.3 Hz, 1H), 1.34 (dddd, J = 13.6, 6.9, 3.3, 1.3 Hz, 1H), 1.19 (s, 3H), 1.04 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 207.8, 67.9, 61.6, 60.5, 33.9, 31.0, 30.4, 27.5, 23.2; [α]D = +75 (c 1.32, CHCl3); HRMS (ESI) Calcld. for C9H14O3 [M+H]+ : 170.0943. Found: 170.0933.
Definitions:
Suspension: A mixture of solid particles in a liquid
Quenched: The use of a base to react with any left over reactants - forcing the reaction to end.
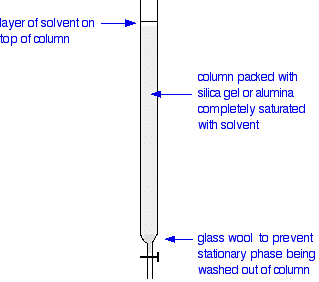
Silica Gel Chromatography: A method of separation that works by extraction different parts of a mixture at a time.
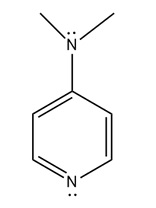
DMAP: 4-Dimethylaminopyridine
ee: Enantiomeric Excess, a measure of purity for chiral substances
Celite: an extremely fine, white powder used as a filtration aid; made up of silicon and oxygen