| Home | Leading Question | Mechanism | Experimental | H-NMR Spectra | References | About Us |
|---|
References
Greetings class! Today we are going to learn about how our reaction, the Sharpless epoxidation reaction, is applied in modern science. Other scientists from around the globe have used this reaction to uncover some interesting transfigurations.
The paper that explains the reaction comes from the following source:
Nagasawa, T.; Shimada, N.; Torihata, M.; Kuwahara, S. Tetrahedron 2010, 66, 4965−4969.
The paper above used the Sharpless epoxidation reaction in the enantioselective formation of idesolide using NaCO3-promoted dimerization. This is similar to our paper, as this group also wishes to make a biological molecule in an enantioselective way using Sharpless epoxidation reaction. The reason why these scientists used Sharpless epoxidation was because they wanted to induce chirality for their molecule. The following reaction used Sharpless epoxidation:
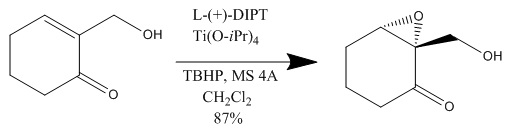
As you can see, the molecule is "transfigured" from an achiral molecule to the chiral product due to the Sharpless epoxidation reaction. Therefore, because they have a chiral reactant, they are able to move forward toward a selected chiral product, idesolide. The other important part of their paper involved dimerization. The group used NaCO3 as a catalyst for dimerization in the following reaction:
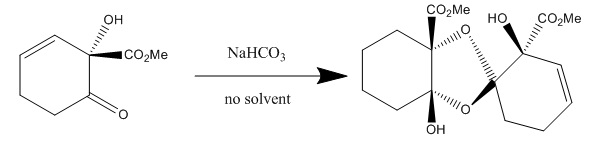
By using 2 equivalents of NaCO3, they were able to increase their yield of this dimer from 0% to 65%.
Three other papers cited the paper above in their own papers. These are "cousins" of our paper:
Perin, G.; Borges, E. L.; Rosa, P. C.; Carvalho, P. N.; Lenardao, E. J. Tetrahedron Letters 2013, 54, 1718-1721.
This group wanted to create a certain type of organoselenium compound, a beta-selenocarbonyl. A beta-selenocarbonyl was used as an intermediate in the creation of idesolide. For this reason, the group decided to cite the paper above.
Kim, T. B.; Kim, H. W.; Lee, M.; Lee, H. H.; Kim, S. H.; Kang, S. K.; Sung, H. S. Tetrahedron Letters 2014,55,5447-5449.
This group wished to make a molecule that is related to idesolide called idescarparide. Both of these molecules are spiro-type (ring structure) compounds used by the deciduous tree species I. polycarpa. Therefore, they are creating similar molecules, and decided to cite the paper.
Griffith, D. R.; Botta, L.; Denis, T. G. S.; Snyder, S. A. J. Org. Chem. 2014, 79, 88-105.
This group was interested in creating a model Yunnaneic acid, which is a caffeine derivative. Similar to how the cited group used NaCO3-promoted dimerization, this group also used dimerization to create their molecule of interest.