Question:
Provide a detailed rationale for the regioselectivity and diastereoselectivity of the Diels-Alder reaction.
The Diels-Alder reaction here proceeds with normal electron demand, so the diene is the nucleophile while the dienophile is the electrophile. Thus, we can analyze the relevant resonance structures of each reactant to understand the regio and stereoselectivity of the reaction.
The compound forms as a single diastereomer, so we will attempt to explain why.
Firstly, the silyl ether on intermediate 6 is conjugated with the double bond. Though it is an electron-withdrawing group inductively, because the alternate resonance structure has closed-shell atoms it functions more as a electron-donating group. The carbon not bonded to the silyl ether gains a partial negative charge.

Nitroethylene, on the other hand, has an electron-withdrawing nitro group both inductively and by resonance. The middle resonance contributor has open shell atoms and contributes less; nevertheless it is important in understanding the partial charges on the atoms. The carbon not bonded to the nitro group gains a partial positive charge. The speed of the reaction is also helped by the gathering of negative charge on the diene.

Now for the conformation:

This is the conformation that produces the expected diastereomer. The regioselectivity can be explained by considering the partial positive charges on the carbons due to the resonance structures. The partially positive carbon on the dienophile lines up with the partially negative carbon on the diene. The speed of the reaction is also increased by the gathering of positive charge on the dienophile.
The ring on the lower face of the diene inhibits approaching of the dienophile from the bottom face, especially because the endo regioselective reaction is most inhibited.
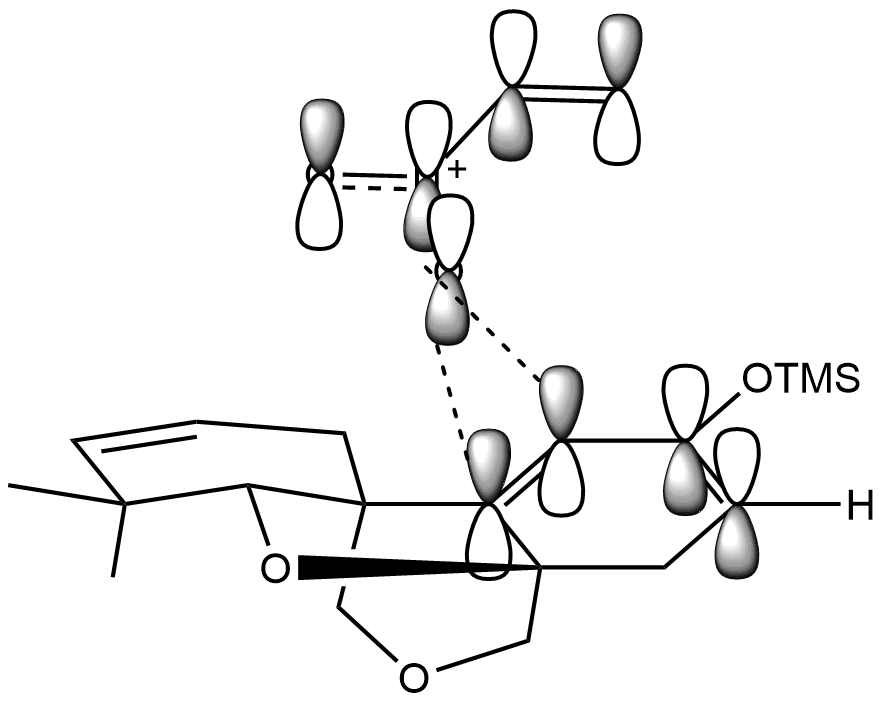 Stereochemically, the Diels-Alder reaction will prefer the endo conformation because the pi system on the nitro group has a positive interaction with the orbitals on the diene, reducing the energy of the transition state. Note that in reality the molecular orbitals are more complicated than shown.
Stereochemically, the Diels-Alder reaction will prefer the endo conformation because the pi system on the nitro group has a positive interaction with the orbitals on the diene, reducing the energy of the transition state. Note that in reality the molecular orbitals are more complicated than shown.