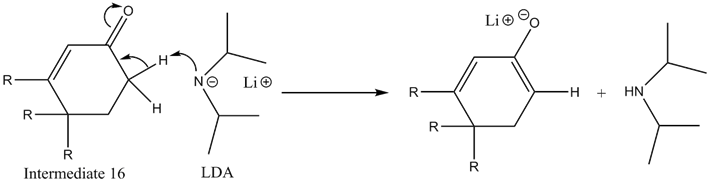
Conversion of Intermediate 15 to Intermediate 6
The first step of this conversion is to form an enolate from the ketone present in Intermediate 15. This is performed by the addition of Lithium Diisopropylamine or LDA. LDA has a pKa of approximately 36, so it can easily deprotonate the beta hydrogens on the sp3 carbon—note that the beta-hydrogen on the other side of the ketone is attached to an sp2 carbon, meaning it would be far less acidic, especially when taking into account the lack of stabilizing resonance structures with that hypothetical conjugate base.
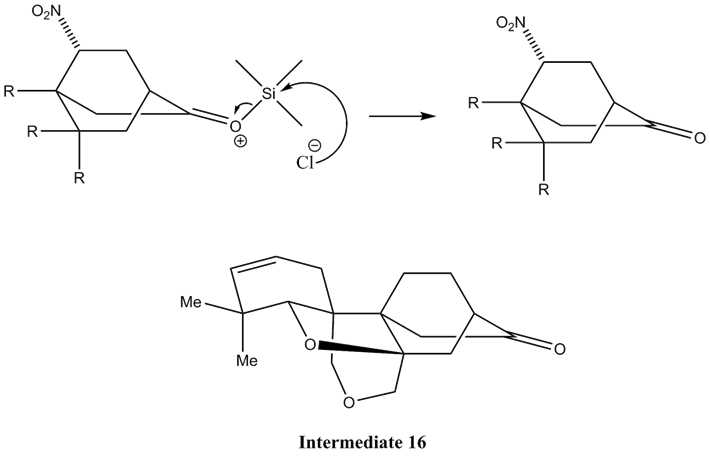
The next step of this conversion would be to protect the possibly reactive oxygen atom in the enolate. This can be accomplished by the introduction of TMSCl or Trimethylsilyl chloride. This forms Intermediate 6.

Diels-Alder Reaction
Now that Intermediate 6 has been formed, now the key Diels-Alder Reaction can take place. Intermediate 6 acts as the diene, and thus nucleophile, while Nitroethylene acts as the dienophile, and thus electrophile. The resonance structures shown below explain the regioselectivity of this Diels-Alder reaction, in particular how the nitro- group ends up on the opposite side from the oxygen group.
The partially positive end opposite the nitro- group in Nitroethylene will align selectively with the partially negative carbon in Intermediate 6, leading to a regioselective alignment of the diene and dienophile.
The stereoselectivity of this Diels-Alder reaction can be rationalized by having the nitroethylene attack from the “front” of the molecule, as shown in the diagram below. The reaction then pursues an endo- formation, so that the many pi-orbitals on the nitro-group can be stabilized by the conjugated pi-system of intermediate 6.
The fourth step uses hydrochloric acid to protonate the double bond, creating a carbocation that is stabilized by the oxygen in the OTMS group, displacing the positive charge up onto the oxygen.
Now for the final step in the conversion from Intermediate 6 to Intermediate 16, the chloride ion substitutes for the oxygen atom and removes the protecting group. This leaves behind Intermediate 16, which completes the key step in the total synthesis of maoecrystal V.