Main page
Mechanism 1-HNMR Experimental References Leading Question About Us SSG Home
The Chairman-Emperor of China commands you:
Draw the structure and mechanism of the major side product that limits the yield of this reaction as discussed by the authors
The mechanism for the formation of the byproduct only deviates from the mechanism for the desired product after the formation of the acyl radical. Thus, AIBN first decomposes into radicals that will act as the catalyst for this reaction just as it did for the formation of the desired product. Likeweise, this decomposition is followed by H atom abstraction from the co-catalyst tris(trimethylsilyl)silane.

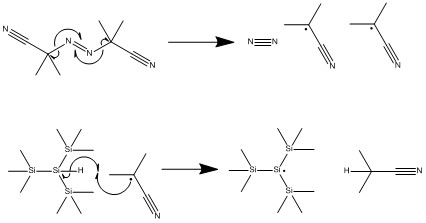

After the formation of the tris(trimethylsilyl)silane radical, the acyl radical is formed just as in the mechanism for the formation of the desired product. However, after the formation of this radical, the mechanism to afford the byproduct diverges.



The acyl radical rearranges to afford an alkyl radical in this step. This decarboxylation step proceeds due to the entropically favored release of carbon dioxide by the system.

In a six-membered-ring-like transition state, the alkyl radical abstracts a Hydrogen from another Carbon atom on the molecule, resulting in the deprotection of an Oxygen and a new alkyl radical stabilized by an aromatic ring. Finally, the resonance-stabilized alkyl radical abstracts a Hydrogen from the protonated catalyst. This reaction proceeds due to the formation of the stable Oxygen protecting group PMB, and reformation of the catalyst. Thus, the catalyst may continue to contribute to the formation of acyl radicals in subsequent reactant molecules.



Leading Question