Main page
Mechanism 1-HNMR Experimental References Leading Question About Us SSG Home
Main Reference:
Lu, P.; Gu, Z.; Zakarian, A. J. Am. Chem. Soc. 2013, 135, 14552-14555.
The paper cited used intramolecular radical cyclization to close a ring in a similar fashion to step 58 in our paper. AIBN was utilized as a catalyst to create an acyl radical that could then react with the double bond in the molecule to perform the cyclization. For this transformation, the authors experimented with a variety of reagents. They finally settled on tris(trimethylsilyl)silane as the co-catalyst that resulted in the most efficient transformation to product 19. This paper also cited that the use of this co-catalyst resulted in the formation of byproduct 24 from radical fragmentation. This supports the byproduct we presented for the formation of compound 59 in our paper because both reactions involve the entropically favored release of carbon dioxide, removal of an oxygen protecting group, and reformation of the catalyst.
Formation of 19 from 18 Formation of stable byproduct 24




Reference 1:
Trost, B. M.; Waser, J.; Meyer, A. J. Am. Chem. Soc. 2008, 130, 16424-16434.
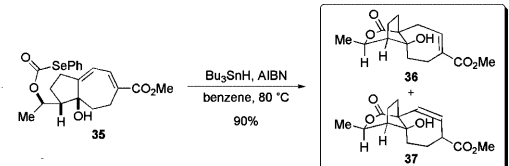
This paper shows the schematic of an intramolecular ring closing. This reaction utilizes the spontaneously decomposing AIBN and a selenium phenyl group to lead to the formation of an acyl radical. This reaction is very similar to how AIBN is used in the transformation from compound 58 to compound 59 in our paper as it utilizes similar reagants.


Reference 2:
Taniguchi, T.; Sugiura, Y.; Zaimoku, H.; Ishibashi, H. Angew. Chem. Int. Ed. 2010, 49, 10154-10157.
This paper contains a useful schematic demonstrating the generation of carbonyl radicals from an initiator or catalyst. A common co-catalyst used to create these radicals is tris(trimethylsilyl)silane; this reagant was also used in the formation of compound 59. These radicals are very reactive and can often undergo intramolecular ring closures with double bonds.



Reference 3:
Begley, M. J.; Ladlow, M.; Pattenden, G. J. Chem. Soc. 1988, 5, 1095-1106.
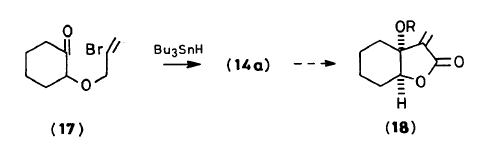
This paper discusses the radicophilicity of a carbonyl group when AIBN is used in conjunction with tributylstannane. This reacts further to undergo an intramolecular ring closing. The paper mentions that low yield is common with these closures, similar to how compound 59 only had a 40% yield. The paper also meantioned the creation of significant byproducts, similar to the formation of the byproduct created alongside compound 59 as seen in the leading question.

References