All of the following reactions were carried out under a chemically inactive atmosphere of dry argon in oven or flame-dried glassware, unless the reaction procedure states otherwise.Tetrahydrofuran (THF) and diethyl ether were distilled from sodium-benzophenone in a continuous still under an atmosphere of argon. Dichloromethane, di-iso-propylamine and triethylamine were distilled from calcium hydride in a continuous still under an atmosphere of argon.
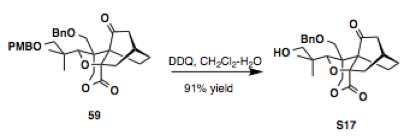
DDQ (25 mg, 0.112 mmol) was added to a solution of the substrate 59 (30.0 mg, 56.0 µmol) in CH2Cl2 (2.5 mL) and H2O (0.2 mL) at 23 °C. After stirring for 1 h, the reaction mixture was diluted with CH2Cl2, quenched with a saturated aqueous NaHCO3 solution, and extracted with CH2Cl2. The combined organic phase was sequentially washed with a saturated aqueous NaHCO3 solution and brine, dried over Na2SO4, concentrated and the residue was purified by column chromatography on silica gel (30% ethyl acetate in hexanes) to deliver the product S17 (21.0 mg, 50.7 µmol, 91%).
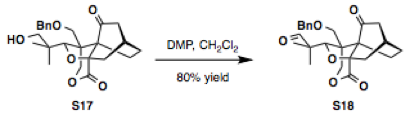
Sodium bicarbonate (0.103 g, 1.23 mmol) and Dess-Martin periodinane (DMP) (0.140 g, 0.328 mmol) were added sequentially to a solution of alcohol S17 (85.0 mg, 0.205 mmol) in CH2Cl2 (10 mL) at 23 °C. After stirring for 1 h, the reaction mixture was diluted with CH2Cl2, quenched with a saturated aqueous NaHCO3 and Na2S2O3 solution, and extracted with CH2Cl2. The combined organic phase was washed with brine, dried over Na2SO4, concentrated and the residue was purified by column chromatography on silica gel (15% ethyl acetate in hexanes) to deliver the product S18 (67.5 mg, 0.163 mmol, 80%).

A solution of n-Butyllithium (2.25 M in hexanes, 0.35 mL, 0.764 mmol) was added to a suspension of CH3Ph3PBr (0.311 g, 0.974 mmol) in THF (5 mL) at 0 °C. After stirring at the same temperature for 15 min, 4 ml of the resulting yellow solution was transferred to a solution of aldehyde S18 (44 mg, 0.107 mmol) in THF (2 mL) at 0 °C. After stirring for 10 min, the reaction was quenched with a saturated aqueous NH4Cl solution and extracted with ethyl acetate. The combined organic phase was washed with brine, dried over Na2SO4, concentrated and the residue was purified by column chromatography on silica gel (15% ethyl acetate in hexanes) to deliver the product 60 (33.5 mg, 81.7 µmol, 76%).
Glossary
Tetrahydrofuran (THF): A five-membered heterocyclic ring consisting of four carbon atoms and one oxygen atom, or a molecule containing this ring. Also the nonpolar aprotic solvent of molecular formula C4H8O containing this ring.
DDQ: This term is an abbreviation for 2,3-dichloro-5,6-dicyanobenzoquinone, a relatively strong oxidant that is often used for dehydrogenation
Saturated: When a solution is saturated, it means that it has the maximum amount of a given compound dissolved in it. Any additional compound added to the solution would not dissolve unless the mixture is heated or otherwise altered.
Dess-Martin Periodinane: Also known as DMP, Dess-Martin Periodinane is a chemical commonly used to oxidize primary and secondary alcohols to aldehydes and ketones, respectively.