
In chapter 2 we saw that if we had –rA as a function of X, [–rA= f(X)] we could size many reactors and reactor sequences and systems.

How do we obtain –rA = f(X)? We do this in two steps 1. Part 1 - Chapter 3 Rate Law – Find the rate as a function of concentration, –rA = k fn (CA, CB …) 2. Part 2 - Chapter 4 Stoichiometry – Find the concentration as a function of conversion CA = g(X) Combine Part 1 and Part 2 to get -rA=f(X) |
We shall set up Stoichiometric Tables using A as our basis of calculation
in the following reaction. We will use the stoichiometric tables to express
the concentration as a function of conversion. We will combine Ci
= f(X) with the appropriate rate law to obtain -rA = f(X).
| Batch System Stoichiometric Table (p.4-2) | top |
| Species | Symbol | Initial | Change | Remaining |
|---|---|---|---|---|
| A | A | |||
| B | B | |||
| C | C | |||
| D | D | |||
| Inert | I | ________ |
------- | ____________ |
| and |
Concentration -- Batch System: |
Constant Volume Batch:
|
Note: if the reaction occurs in the liquid phase or if a gas phase reaction occurs in a rigid (e.g., steel) batch reactor |
|
| Then | |
| etc. | |
if ![]() then
then
|
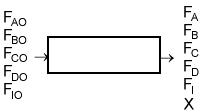
| Flow System Stoichiometric Table (p.4-9) | top |
 |
||||
| Species | Symbol | Reactor Feed | Change | Reactor Effluent |
|---|---|---|---|---|
| A | A | |||
| B | B | |||
| C | C | |||
| D | D | |||
| Inert | I | ________ |
------- | ____________ |
Where:
| and |
Concentration -- Flow System:
|
Liquid Phase Flow System:
|
|
|
If the rate of reaction were -rA = kCACB then
we would have ![]()
This
gives us -rA = f(X). Consequently, we can use the methods
discussed in Chapter 2 to size a large number of reactors, either alone
or in series.
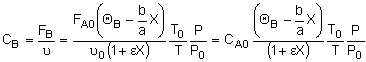 |
|
etc. Again, these equations give us information about -rA = f(X), which we can use to size reactors.
then |
|
|
with
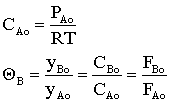
![]() Rate Law in terms of Partial Pressures
Rate Law in terms of Partial Pressures
Calculating the equilibrium conversion for gas phase reaction
Consider the following elementary reaction with KC and = 20 dm3/mol and CA0 = 0.2 mol/dm3. Pure A fed. Calculate the equilibrium conversion, Xe, for both a batch reactor and a flow reactor.
Solution
At equilibrium
Stoichiometry
Batch
Species Initial Change Remaining A NA0 -NA0X NA = NA0(1-X) B 0 +NA0X/2 NB = NA0X/2 NT0 = NA0 NT = NA0 - NA0X/2 Constant Volume V = V0
Solving
Flow
Species Fed Change Remaining A FA0 -FA0X FA = F A0(1-X) B 0 +FA0X/2 FB = F A0X/2 FT0 = F A0 FT = F A0 - F A0X/2
Recall
The following humorous video on limiting reactants was made by Professor Lane's 2008 Chemical Reaction Engineering class at the University of Alabama, Tuscaloosa.
You may access the video by connecting to the internet and clicking the image below

* All chapter references are for the 1st Edition of the text Essentials of Chemical Reaction Engineering .