
H-NMR Correlation

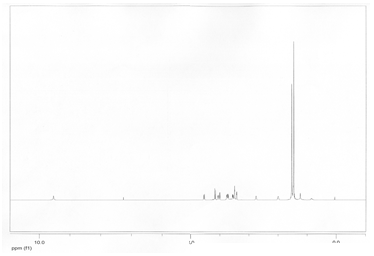
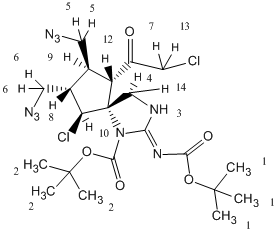
Compound 18:
H-NMR Peak |
Corresponding H and Explanation |
1.47 |
2- This group is a singlet which is supported by the fact that they are only neighbors to itself. Also, this peak corresponds to 9 H’s, which there are 9 H’s that are all the same. This group is a little less deshielded than group 1 because group 1 has a N=C fairly close to it. This makes group 1 slightly more deshielded than group 2. |
1.49 |
1- This group is a singlet which is supported by the fact that they are only neighbors to itself. Also, this peak corresponds to 9 H’s, which there are 9 H’s that are all the same. This group is slightly more deshielded than group 2 because this group has an N=C fairly close to it while group 2 has a N-C the same distance away. This makes group 2 slightly more deshielded. |
2.05-1.99 |
9- This is deshielded by the fact that it is connected to a tertiary carbon. It has 4 3-bond neighbors which means it should be a multiplet. This is supported by the data. It is fairly far away from the other electronegative atoms which makes it less deshielded than most of the other H’s. |
2.80-2.75 |
8- This carbon is attached to a terciary carbon. However, it is more deshielded than 9 because it is close to the electronegative Cl. Also, it has 4 3-bond neighbors which explains why it is a multiplet on the NMR. |
3.44 |
4- This carbon is attached to a tertiary carbon. However, it is more deshielded than 9 and 8 because it is closer to the electronegative N and could also be slightly affected by the nitrogen 3 bonds away. |
3.51-3.50 |
5- These two H’s are fairly deshielded by the N3 group that is near them. They could have H-bonding with the nitrogens which would explain why it is a multiplet with only one 3-bond neighbor. This group is less deshielded than group 6 which is very similar to it because group 6 has a very electronegative Cl next to it whereas group 5 does not. This implies that group 6 will be more deshielded than group 5. |
3.56 |
14- This is more deshielded than 4 because it is in closer proximity to the C=O group, and the N going into the page. It can have H-bonding with both of these, making it more deshielded than 4. Also, being close to the other N makes it deshielded as well. |
3.79-3.72 |
6- These two H’s are fairly deshielded by the N3 group that is near them. They could have H-bonding with the nitrogens which would explain why it is a multiplet with only one 3-bond neighbor. Also, this group is more deshielded than group 5 which is very similar to it because it is closer to an electronegative Cl which makes it a little more deshielded than group 5. |
4.01 |
7- This H is next to a Cl which makes it about a 3.5, and it is further deshielded by the C=O near it. It is different from 13 because there could be slight H-bonding between 13 and the oxygen near it. This makes 13 a 2-bond neighbor which explains why it is a doublet. |
4.07 |
13- This H is next to a Cl which makes it about 3.5, and it is further deshielded by the C=O near it. It is different from 7 because there could be slight H-bonding between this H and the oxygen near it. This explains why 13 is slightly more deshielded than 7 because it has more interaction with the oxygen. Also, 7 is different from 13 which makes 7 a 2-bond neighbor and explains why 13 is a doublet. |
4.17 |
12- This carbon is attached to a tertiary carbon. It is more deshielded than 9, 8, and 4 because it is close to the Cl and the N. It is also affected by the C=O one bond away from the carbon it is attached to.This makes it much more deshieled than the others. |
4.56 |
10- This H is attached to a carbon that is attached to a secondary carbon and a Cl. This makes it very deshielded. Also, the nitrogen 3 bonds away also could affect it slightly and make it more deshielded. |
9.55 |
3- This H is connected to a very electronegative nitrogen, making it much more deshielded than all of the other H’s because they are all attached to much less electronegative carbons. |


Compound 19:
H-NMR |
Correlation and Explanation |
1.54-1.38 |
1- All of these H’s are in the same group because there are so many of them and they are so close together that they make a jumbled mess. This makes it impossible to tell one group from another. Also, they are not very deshielded because all of them are sp3 CH3 groups attached to another carbon. |
2.31-2.24 |
2- This is deshielded by the fact that it is connected to a tertiary carbon. It has 4 3-bond neighbors which means it should be a multiplet. This is supported by the data. It is fairly far away from the other electronegative atoms which makes it less deshielded than most of the other H’s. |
2.49-2.42 |
5- This carbon is attached to a terciary carbon. However, it is more deshielded than 2 because it is close to the electronegative Cl. Also, it has 4 3-bond neighbors which explains why it is a multiplet on the NMR. |
3.35-3.38 |
7- These two H’s are fairly deshielded by the N3 group that is near them. They could have H-bonding with the nitrogens which would explain why it is a multiplet with only one 3-bond neighbor. This group is less deshielded than group 8 which is very similar to it because group 8 has a very electronegative Cl next to it whereas group 7 does not. This implies that group 8 will be more deshielded than group 7. |
3.58-3.52 |
8- These two H’s are fairly deshielded by the N3 group that is near them. Also, this group is more deshielded than group 7 which is very similar to it because it is closer to an electronegative Cl which makes it a little more deshielded than group 7. |
3.63 |
10- This is more deshielded than 9 because it is in closer proximity to the C=C group, and the N going into the page. It can have H-bonding with both of these, making it more deshielded than 9. Also, being close to the other N makes it deshielded as well. |
4.00 |
6- This carbon is attached to a tertiary carbon. It is more deshielded than 9, 8, because it is close to the Cl and the N. It is also affected by the C=C one bond away from the carbon it is attached to.This makes it much more deshieled than the others. |
4.33 |
4- This H is attached to a carbon that is attached to a secondary carbon and a Cl. This makes it very deshielded. Also, the nitrogen 3 bonds away also could affect it slightly and make it more deshielded. |
7.34 |
3- This carbon is much more deshielded than all of the others because it is connected to an sp2 carbon whereas all of the others are connected to sp3 carbons. Also, it is a singlet which makes sense because it has no 3-bond neighbors. |
